- Details
- Description
-
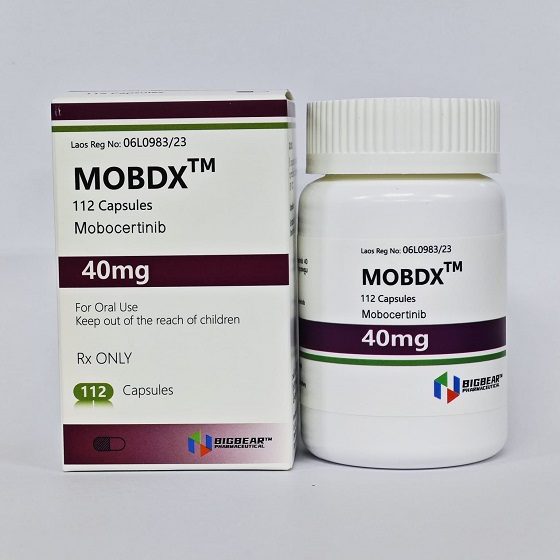
Packaging Size112C/bottle
-
Strength40mg
-
CompositonMobocertinib
-
TreatmentNon-small cell lung cancer (NSCLC) with EGFR bearing mutations in the exon 20 region
-
FormCapsule
-
BrandMOBODX
-
Quantity Unit40mg*112C/Bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
Mobocertinib is a small molecule tyrosine kinase inhibitor. Its molecular target is epidermal growth factor receptor (EGFR) bearing mutations in the exon 20 region.
Non-Small Cell Lung Cancer
Indicated for locally advanced or metastatic non–small cell lung cancer (NSCLC) in adults with epidermal growth factor receptor (EGFR) exon 20 insertion mutations and whose disease has progressed on or after platinum-based chemotherapy
160 mg PO qDay
Continue until disease progression or unacceptable toxicity
Dosage Modifications
Dose reductions for adverse reactions
- First dose reduction: 120 mg PO qDay
- Second dose reduction: 80 mg PO qDay
Black Box Warnings
QTc prolongation and torsades de pointes
- Life-threatening heart rate–corrected QTc prolongation, including torsades de pointes, may occur
- Monitor QTc and electrolytes periodically during treatment
- Increase monitoring frequency in patients with risk factors for QTc prolongation (eg, congenital long QT syndrome, heart disease, electrolyte abnormalities)
- Avoid coadministration with drugs that may further prolong the QTc interval (eg, drugs known to prolong QTc interval, strong or moderate CYP3A inhibitors)
- Withhold, reduce dose, or permanently discontinue treatment based on the severity of QTc prolongation