- Details
- Description
-
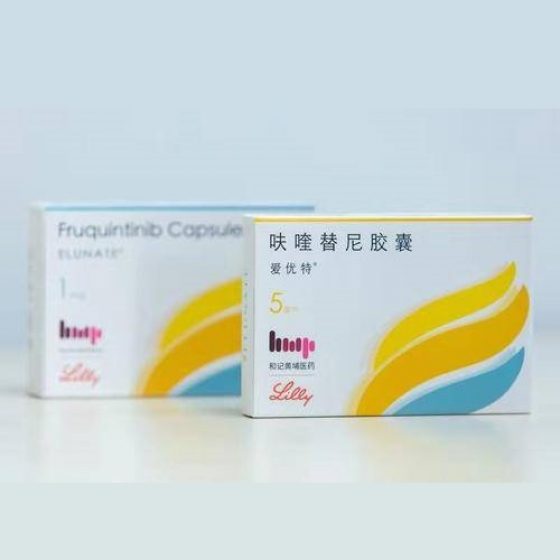
Packaging Size7capsules/Box
-
Strength1mg,5mg
-
CompositonFruquintinib (呋喹替尼)
-
TreatmentMetastatic colorectal cancer
-
FormCapsule
-
BrandFruzaqla (爱优特,ELUNATE)
-
Quantity Unit(1mg&5mg)*7capsules/Box
-
ManufacturerHUTCHMED Biopharmaceutical Company,china
About Fruquintinib
Fruquintinib (Fruzaqla) sold under the brand name 爱优特 in china, is an oral, highly selective, small molecule VEGF receptor inhibitor. Because of the kinase selectivity of Fruzaqla, the risk of off-target toxicity is less than the risk with multikinase inhibitors, including regorafenib.
- Fruquintinib продается под торговой маркой Fruzaqla в США
- Fruquintinib продается под торговой маркой ELUNATE в Гонконге
- Fruquintinib продается под торговой маркой 爱优特 в Китае
Approval
November 9, 2023
The FDA has approved fruquintinib (Fruzaqla) to treat adults with metastatic colorectal cancer who received prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-VEGF therapy, and an anti-EGFR therapy for cases that are RAS wild-type and medically appropriate.
June 21, 2024
The European Commission (“EC”) that it has approved FRUZAQLA® (fruquintinib) as a monotherapy indicated for the treatment of adult patients with metastatic colorectal cancer (“CRC”) who have been previously treated with available standard therapies, including fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapies, anti-VEGF agents, and anti-EGFR agents, and who have progressed on or are intolerant to treatment with either trifluridine-tipiracil or regorafenib.
September 24, 2024
The Japanese Ministry of Health, Labour and Welfare to manufacture and market FRUZAQLA Capsules 1mg/5mg (generic name: fruquintinib), a selective oral inhibitor of vascular endothelial growth factor receptor (VEGFR) -1, -2 and -3, for the treatment of advanced or recurrent colorectal cancer (CRC) that is neither curable nor resectable and that has progressed after chemotherapy.