- Details
- Description
-
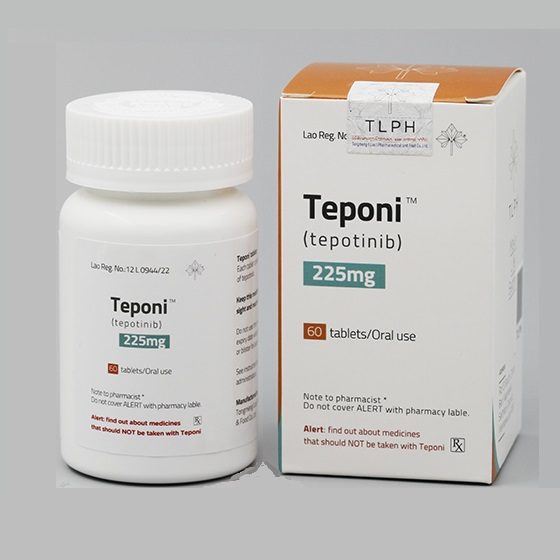
Packaging Size60T/bottle
-
Strength225mg
-
CompositonTepotinib
-
TreatmentNon-small cell lung cancer (NSCLC) with exon 14 skipping - it inhibits MET phosphorylation
-
FormTablet
-
BrandTeponi
-
Quantity Unit225mg*60T/bottle
-
ManufacturerTongmeng (Lao) Pharmaceutical & Food Co., Ltd.(TLPH)
Tepotinib is a kinase inhibitor directed against MET, including variants with exon 14 skipping - it inhibits MET phosphorylation and subsequent downstream signaling pathways in order to inhibit tumor cell proliferation, anchorage-independent growth, and migration of MET-dependent tumor cells.
Non–Small Cell Lung Cancer
Indicated for metastatic non–small cell lung cancer (NSCLC) in adults harboring mesenchymal-epithelial transition (MET) exon 14 (ex14) skipping alterations
450 mg PO qDay
Continue until disease progression or unacceptable toxicity
Dosage Modifications
Recommended dose reduction to manage adverse reactions: 225 mg PO qDay
Permanently discontinue if unable to tolerate 225 mg PO qDay
Interstitial lung disease (ILD)/ pneumonitis
- Suspected ILD (any grade): Withhold
- Confirmed ILD: Permanently discontinue
Increased AST/ALT and/or total bilirubin
-
Grade 3 increased AST/ALT without increased total bilirubin
- Withhold until recovery to baseline ALT/AST
- If recovered to baseline ≤7 days, resume at same dose; otherwise, resume at a reduced dose
-
Grade 3 increased total bilirubin without concurrent increased AST/ALT
- Withhold until recovery to baseline total bilirubin
- If recovered to baseline ≤7 days, resume at reduced dose; otherwise, permanently discontinue
-
Permanently discontinue
- Grade 4 increased ALT/AST without increased total bilirubin
- ALT/AST >3x ULN with total bilirubin >2x ULN without cholestasis or hemolysis
- Grade 4 increased total bilirubin without concurrent increased AST/ALT
Other adverse reactions
- Grade 2: Maintain dose; if intolerable, consider withholding until resolved, then resume at reduced dose
- Grade 3: Withhold until resolved, then resume at reduced dose
- Grade 4: Permanently discontinue
Renal impairment
- Mild-to-moderate (CrCl 30-89 mL/min): No dosage adjustment necessary
- Severe (CrCl <30 mL/min): Recommended dose not established
Hepatic impairment
- Mild-to-moderate (Child-Pugh Class A or B): No dosage adjustment necessary
- Severe (Child-Pugh Class C): Pharmacokinetics and safety not studied