- Details
- Description
-
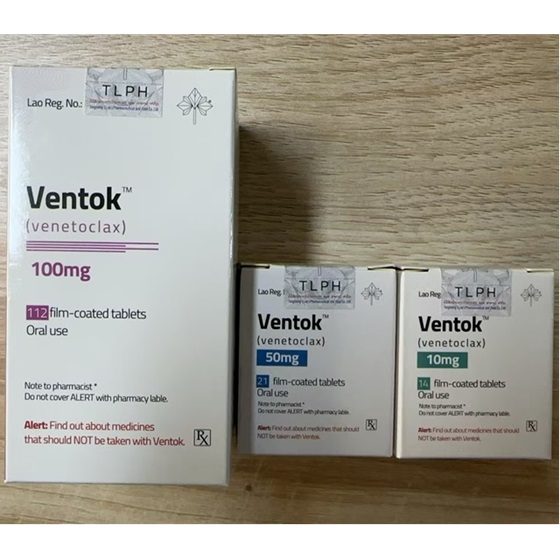
Packaging Size10mg&50mg&100mg*14tablets&21tablets&114tablets/Box
-
Strength10mg&50mg&100mg
-
CompositonVenetoclax
-
TreatmentChronic lymphocytic leukemia(CLL), Small lymphocytic lymphoma(SLL), Acute myeloid leukemia(AML)
-
FormTablet
-
BrandVentok
-
Quantity Unit14tablets&21tablets&114tablets/Box
-
ManufacturerTongmeng (Lao) Pharmaceutical & Food Co., Ltd.(TLPH)
Brand name: Ventop.
Generic name: Venetoclax
[Indications]
Venetoclax is a bcl-2 inhibitor for the treatment of patients with 17p-deficient chronic lymphocytic leukemia (CLL) who have received at least one previous treatment and are tested by an FDA-approved test. This indication was approved under accelerated approval based on the overall response rate, and continued approval of this indication may depend on the description and confirmation of clinical benefits in a confirmatory trial.
[How to take the drug]
1. Therapy period
● Venetoclax was initially treated once daily at 20mg for 7 days, followed by an incremental weekly schedule to the recommended daily dose of 400mg.
● Venetoclax tablets should be taken orally with food and water once a day.Do not chew, crush or destroy the tablets.
● Prevention of tumor deposition syndrome.
2. Dosage form
● Tablets, 10mg
3. Contraindications
Combination of Venetoclax and CYP3A in the initial and increasing dose stages is contraindicated.
[Warnings and precautions]
● Tumor lysis syndrome (TLS) : prediction of TLS: assessment of risk in all patients.Administer antihyperuricemia medication in advance and ensure proper hydration. Stronger measures (frequent monitoring of intravenous hydration, hospitalization) were applied when the overall risk increased.
●Neutropenia: monitor the blood count and signs of infection for clinically appropriate management.
●Vaccination: do not give live attenuated vaccines before, during, or after Venetoclax treatment.
●Embryo-fetal toxicity: possible embryo-fetal toxicity.Women with reproductive potential are advised to be at risk to the fetus and to use effective contraception during treatment.
[Side-effect]
● The most common adverse reactions (220%) included: neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia and fatigue.
● Common laboratory abnormalities (≥25%) include creatinine, increased mean red blood cell volume;Hemoglobin, lymphocytes, absolute neutrophils, thrombocytopenia.
[How to store the drug]
● Store the medicine lower than 30℃.