- Details
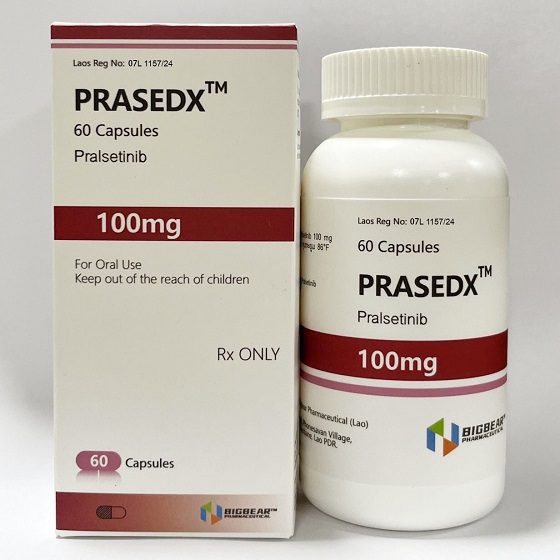
- Description
-
Packaging Size60c/bottle
-
Strength100mg
-
CompositonPralsetinib
-
TreatmentRET mutation-positive medullary thyroid cancer , RET fusion-positive differentiated thyroid cancer,RET fusion-positive non-small cell lung cancer (NSCLC)
-
FormCapsule
-
BrandPRASEDX
-
Quantity Unit100mg*60c/bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
About Pralsetinib
Pralsetinib is a tyrosine kinase inhibitor. It is taken by mouth.
Pralsetinib is a medication approved for RET mutation-positive medullary thyroid cancer (MTC) and RET fusion-positive differentiated thyroid cancer (DTC) refractory to radioactive iodine (RAI) therapy.
Pralsetinib is indicated for the treatment of adults with metastatic RET fusion-positive non-small cell lung cancer (NSCLC) .
Non-Small Cell Lung Cancer
Indicated for metastatic rearranged during transfection (RET) gene-positive non-small cell lung cancer (NSCLC)
400 mg PO qDay on an empty stomach
Continue until disease progression or until unacceptable toxicity
Medullary Thyroid Cancer
Indication was withdrawn in the U.S. by manufacturer on July 10, 2023
The decision was made to remove the indication after the confirmatory trial could not fulfill the postmarketing requirement
Thyroid Cancer
Indicated for advanced or metastatic RET-fusion positive thyroid cancer in adults who require systemic therapy and are radioactive iodine-refractory (if radioactive iodine is appropriate)
400 mg PO qDay
Continue until disease progression or until unacceptable toxicity
Dosage Modifications
Dosage modifications for adverse reactions
- First dose reduction: 300 mg PO qDay
- Second dose reduction: 200 mg PO qDay
- Third dose reduction: 100 mg PO qDay
- Unable to tolerate 100 mg qDay: Permanently discontinue
Interstitial lung disease (ILD)/pneumonitis
- Grade 1 or 2: Withhold until resolution; resume at reduced dose
- Grade 3 or 4 or recurrent ILD/pneumonitis: Permanent discontinue
Hypertension
- Grade 3: Withhold for persistent Grade 3 hypertension despite optimal antihypertensive therapy; resume at reduced dose once hypertension controlled
- Grade 4: Discontinue
Hepatoxicity
- Grade 3 or 4: Withhold and monitor AST/ALT once weekly until resolution to Grade ≤1
- Resume at reduced dose
- If Grade ≥3 hepatotoxicity recurs, discontinue
Hemorrhagic events
- Grade 3 or 4: Withhold until recovery to baseline or Grade ≤1
- Discontinue for severe or life-threatening hemorrhagic events
Other adverse reactions
- Grade 3 or 4: Withhold until recovery to Grade ≤2; resume at reduced dose
- Recurrent Grade 4: Permanently discontinue
Strong CYP3A4 inhibitors or combined P-gp and strong CYP3A4 inhibitors
- Avoid coadministration
-
Dosage modification if unable to avoid combined P-gp and strong CYP3A4 inhibitors
- If current pralsetinib dose is 300 or 400 mg qDay, reduce to 200 mg qDay
- If current pralsetinib dose is 200 mg qDay, reduce to 100 mg qDay
- After inhibitor has been discontinued for 3-5 elimination half-lives, resume at dose taken before initiating combined P-gp and strong CYP3A inhibitor
Strong CYP3A4 inducers
- Avoid coadministration
- If unable to avoid, double current pralsetinib dose starting on Day 7 of coadministration with strong CYP3A inducer
- After inducer has been discontinued for at least 14 days, resume pralsetinib at dose taken before initiating strong CYP3A inducer
Renal impairment
- Mild-to-moderate (CrCl 30-89 mL/min): No dosage adjustment necessary
- Severe (CrCl <15 mL/min): Not studied
Hepatic impairment
- Mild (total bilirubin less than or equal to ULN and AST > ULN or total bilirubin > 1 to 1.5 × ULN and any AST), moderate (total bilirubin > 1.5 to 3 × ULN and any AST) or severe (total bilirubin > 3 × ULN and any AST): No dosage adjustment necessary