- Details
- Description
-
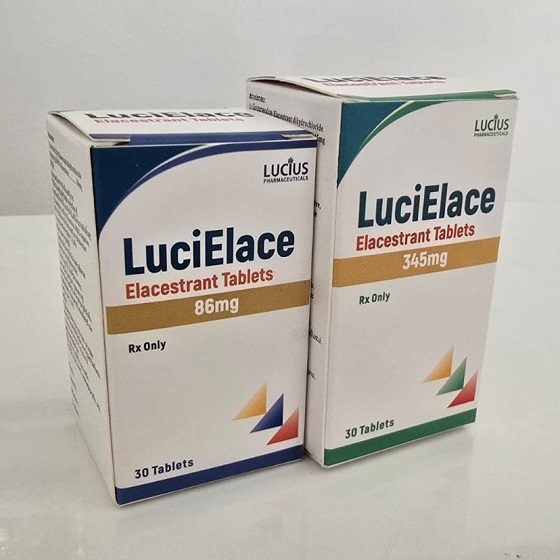
Packaging Size30t/bottle
-
Strength86mg&345mg
-
CompositonElacestrant
-
TreatmentER-positive, HER2-negative, ESR1-mutated, advanced or metastatic breast cancer
-
FormTablet
-
BrandLuciElace
-
Quantity Unit86mg*30t/Box&345mg*30t/Box
-
ManufacturerLucius Pharmaceuticals (Lao) Co.,Ltd
About Elacestrant
About Elacestrant is a selective estrogen receptor degrader (SERD) used in the treatment of breast cancer. Elacestrant is an antiestrogen that acts as an antagonist of estrogen receptors, which are the biological targets of endogenous estrogens like estradiol.
Elacestrant is indicated for the treatment of postmenopausal women or adult men with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, ESR1-mutated, advanced or metastatic breast cancer with disease progression following at least one other line of endocrine therapy.
Breast Cancer
Indicated for men or postmenopausal women with estrogen receptor positive (ER+), HER2-negative, ESR1-mutated advanced or metastatic breast cancer with disease progression following at least 1 line of endocrine therapy
345 mg PO qDay with food until disease progression or unacceptable toxicity
Dosage Modifications
Dose reduction recommendations for adverse reactions
- First dose reduction: 258 mg qDay
- Second dose reduction: 172 mg qDay
- Permanently discontinue if dose reduction <172 mg/day required
Modifications for adverse reactions
- Grade 1: Continue at current dose level
- Grade 2: Consider dose interruption until recovery to Grade ≤1 or baseline, then resume at same dose
-
Grade 3
- Interrupt dose until recovery to Grade ≤1 or baseline, then resume at next lower dose
- If Grade 3 toxicity recurs, interrupt until recovery to Grade ≤1 or baseline, then resume elacestrant reduced by another dose level
-
Grade 4
- Interrupt dose until recovery to Grade ≤1 or baseline, then resume at next lower dose
- Permanently discontinue if Grade 4 or intolerable adverse reaction recurs
Hepatic impairment
- Mild (Child-Pugh A): No dosage adjustment recommended
- Moderate (Child-Pugh B): Reduce dose to 258 mg qDay
- Severe (Child-Pugh C): Avoid use; not studied