- Details
- Description
-
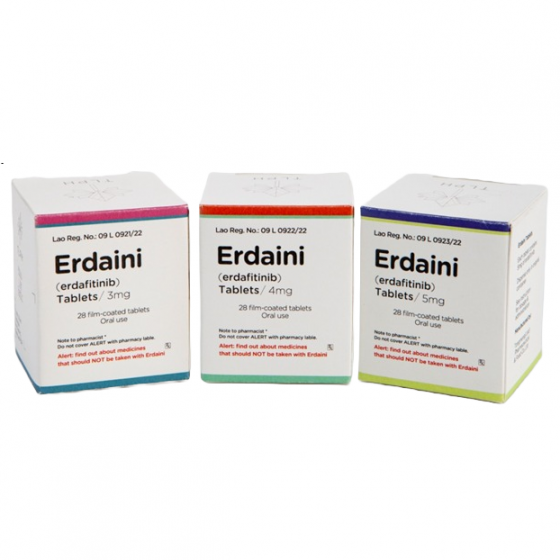
Packaging Size28tablets/Bottle
-
Strength3mg&4mg&5mg
-
CompositonErdafitinib
-
Treatmentused to treat urothelial carcinoma (bladder cancer) that has spread or cannot be removed by surgery
-
FormTablet
-
BrandErdaini
-
Quantity Unit3mg&4mg&5mg*28t/Bottle
-
ManufacturerTongmeng (Lao) Pharmaceutical & Food Co., Ltd.(TLPH)
Erdafitinib is a fibroblast growth factor receptor tyrosine kinase inhibitor used to treat locally advanced or metastatic urothelial carcinoma.
In early April of 2019, the US FDA approved Janssen Pharmaceutical Companies' brand name Balversa (erdafitinib) as the first-ever fibroblast growth factor receptor (FGFR) kinase inhibitor indicated for patients with locally advanced or metastatic urothelial carcinoma, with susceptible FGFR3 or FGFR2 genetic alterations, that has progressed during or following platinum-containing chemotherapy, including within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy . At the same time, the FDA also approved the therascreen FGFR RGQ RT-PCR Kit (Qiagen) for utilization as a companion diagnostic with erdafitinib for selecting patients for the indicated therapy .
Erdafitinib's innovation lies in the fact that it is the first personalized treatment targeting susceptible FGFR genetic alterations for patients with metastatic bladder cancer, which demonstrates the design of erdafitinib in developing more personalized and precision medicines with the capacity to target cancer treatment to a patient's specific genetic mutation . Considering urothelial cancer is statistically the fourth most common kind of cancer in the world , the introduction of erdafitinib offers a welcome new option in the ever-expanding therapeutic tool kit to treat such prevalent medical conditions.
Nevertheless, although erdafitinib was granted Breakthrough Therapy designation and Accelerated Approval from the FDA so as to allow the agency to focus on and expedite the approval process for a medication indicated for a serious condition that fills an unmet medical need using clinical trial data that is believed to predict a genuine clinical benefit for patients with the given condition, such designations mean further ongoing clinical trials are necessary to confirm the clinical benefit of erdafitinib going forward .