- Details
- Description
-
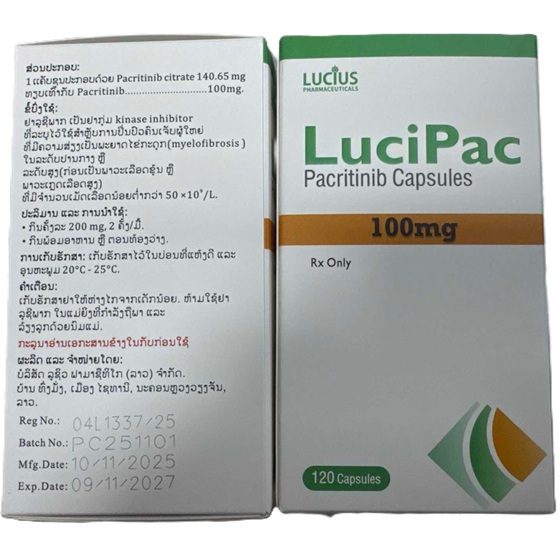
Packaging Size120c/bottle
-
Strength100mg
-
CompositonPacritinib
-
TreatmentMyelofibrosis
-
Formcapsule
-
BrandLuciPac
-
Quantity Unit100mg*120c/box
-
ManufacturerLucius Pharmaceuticals (Lao) Co.,Ltd
About Pacritinib
Pacritinib is a medication used to treat adults with intermediate- or high-risk myelofibrosis (MF), a bone marrow disorder, especially those with low platelet counts. It's a kinase inhibitor that works by blocking the activity of abnormal proteins that signal cancer cells to multiply, slowing or stopping the spread of cancer cells.
Myelofibrosis
Indicated for adults with intermediate or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis (MF) with a platelet count <50 x 109/L
200 mg PO BID
Dosage Modifications
Dose reductions for adverse reactions
- First dose reduction: Reduce to 100 mg BID
- Second dose reduction: Reduce to 100 mg qDay
- Unable to tolerate 100 mg qDay: Discontinue treatment
Planned surgical procedures or other interventions
- Discontinue 7 days before elective surgery or invasive procedures, owing to risk of hemorrhage, and restart only after hemostasis is assured
Diarrhea
- New onset: Initiate antidiarrheal medications; encourage adequate oral hydration
-
Grade 3 or 4
- Defined as increase of ≥7 stools/day over baseline, or hospitalization indicated, or severe increase in ostomy output over baseline, or if limiting self-care
- Hold until diarrhea resolves to Grade ≤1 or baseline (increase of <4 stools/day or mild increase in ostomy output compared with baseline), then restart at last given dose
- Intensify antidiarrheal regimen and provide fluid replacement
- If recurs, hold until diarrhea resolves to Grade ≤1 or baseline, then restart at 50% of last given dose
- Concomitant antidiarrheal treatment is required if restarting
Thrombocytopenia
- In clinically significant worsening of thrombocytopenia lasting >7 days
- Hold therapy until resolution; restart at 50% of last given dose once resolved
- If recurs, hold therapy until resolution, then restart at 50% of last given dose
Hemorrhage
- Moderate bleeding (intervention indicated): Hold until hemorrhage resolves, then restart at last given dose; if recurs, hold until resolution, then restart at 50% of last given dose
- Severe bleeding (transfusion, invasive intervention, or hospitalization indicated): Hold until hemorrhage resolves, then restart at 50% of last given dose; if recurs, discontinue therapy
- Life-threatening bleeding (urgent intervention indicated): Discontinue therapy
QTc prolongation
- QTc prolongation >500 msec or >60 msec from baseline: Hold until QTc interval resolves to ≤480 msec or baseline within 1 week, then restart at same dose
- If time to resolution >1 week, restart at reduced dose
Renal impairment
- eGFR ≥30 mL/min: No dosage adjustment necessary
- eGFR <30 mL/min: Avoid use
Hepatic impairment
- Mild (Child-Pugh A): Decreases AUC by 8.5%; no dosage adjustment necessary
- Moderate or severe (Child-Pugh B or C): Avoid use; decreases AUC by 36% and 45% respectively