- Details
- Description
-
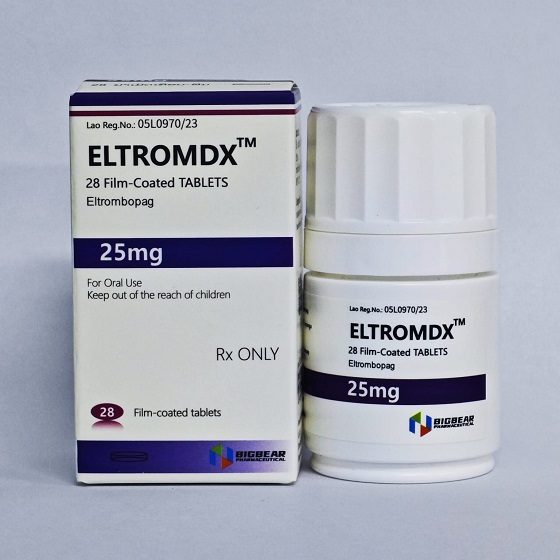
Packaging Size28T/bottle
-
Strength25mg
-
CompositonEltrombopag
-
TreatmentChronic Immune Thrombocytopenia (ITP),Severe Aplastic Anemia,Chronic Hepatitis C-associated Thrombocytopenia
-
FormTablet
-
BrandELTROMDX
-
Quantity Unit25mg*28T/bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
Eltrombopag is used to treat thrombocytopenia (a low blood platelet count) in adults and children with chronic immune thrombocytopenic purpura that did not get better with other treatment.
Chronic Immune Thrombocytopenia (ITP)
Indicated in patients with insufficient response to corticosteroids, immunoglobulins, or splenectomy
Use only in patients with ITP whose clinical condition increases bleeding risk
Initial: 50 mg PO qDay
Maintenance: Adjust dose to achieve and maintain platelet count (Plt) >50 x 10^9/L to reduce risk of bleeding; not to exceed 75 mg/day
Chronic Hepatitis C-associated Thrombocytopenia
Indicated to allow initiation and maintenance of interferon-based therapy
Initial: 25 mg PO qDay
Adjust dose in 25 mg increments q2weeks PRN to achieve target platelet count required to initiate/maintain antiviral therapy with pegylated interferon and ribavirin; not to exceed 100 mg/day
During antiviral therapy, adjust dose to avoid dose reductions of peginterferon
Severe Aplastic Anemia
First-line therapy
- First-line treatment, in combination with standard immunosuppressive therapy, for patients with severe aplastic anemia (SAA)
- Initial dose: 150 mg PO qDay for 6 months
- Do not exceed initial dose; total duration is 6 months
Refractory SAA
- Indicated for SAA in patients who fail to respond adequately to at least 1 prior immunosuppressive therapy
- Initial dose: 50 mg PO qDay
- Adjust dose in 50-mg increments q2Weeks PRN to achieve target Plt ≥50 x 10^9/L as necessary; not exceed 150 mg/day; may take up to 16 weeks for hematologic response