- Details
- Description
-
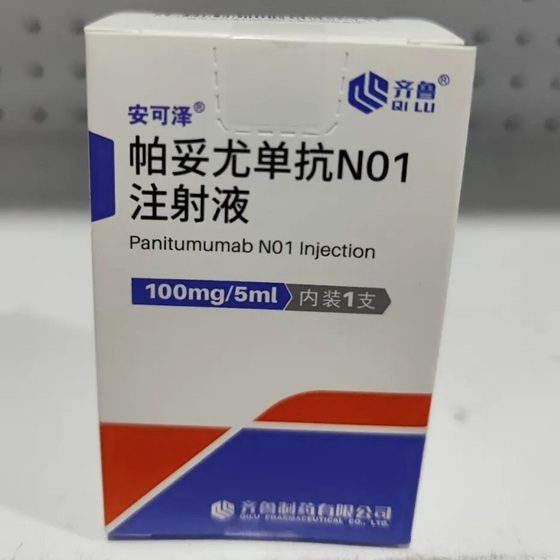
Packaging Size1 bottle
-
Strength100mg/5ml
-
CompositonPanitumumab
-
TreatmentColorectal Cancer
-
FormInjection
-
Brand安可泽®
-
Quantity Unit100mg/5ml/Box
-
ManufacturerQilu pharma,china
About Panitumumab
Panitumumab is a fully human monoclonal antibody specific to the epidermal growth factor receptor (also known as EGF receptor, EGFR, ErbB-1 and HER1 in humans).
Colorectal Cancer
RAS wild-type metastatic colorectal cancer (mCRC)
-
Single-agent therapy
- Indicated for treatment of wild-type RAS mCRC following disease progression after prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy
- 6 mg/kg IV every 14 days until disease progression or unacceptable toxicity
-
Combination therapy
- Indicated in combination with FOLFOX as first-line therapy for wild-type RAS mCRC
- 6 mg/kg IV every 14 days until disease progression or unacceptable toxicity
KRAS G12C-mutated mCRC
- Indicated in combination with sotorasib for treatment of KRAS G12C-mutated mCRC in adults who received prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy
- 6 mg/kg IV every 14 days until disease progression, unacceptable toxicity, or until sotorasib is withheld or discontinued
- Refer to sotorasib prescribing information for recommended sotorasib dosing information
-
Limitations of use
- Not indicated for treatment of RAS-mutant mCRC unless used in combination with sotorasib in KRAS G12C-mutated mCRC
- Not indicated for treatment of mCRC if RAS mutation status unknown
Dosage Modifications
Combination therapy with sotorasib
- If therapy with sotorasib is temporarily withheld or permanently discontinued, temporarily withhold or permanently discontinue panitumumab
- Refer to the sotorasib prescribing information for dose modifications for adverse reactions associated with use of sotorasib.
Infusion reactions
- Mild-moderate (grade 1-2): Reduce infusion rate by 50%
- Severe (grade 3-4): Terminate infusion; permanently discontinue depending on severity and/or persistence
Dermatologic reactions
- First occurrence of grade 3 dermatologic reaction: Withhold 1 to 2 doses; if reaction improves to
- Second occurrence of grade 3 dermatologic reaction: Withhold 1 to 2 doses; if reaction improves to
- Third occurrence of grade 3 dermatologic reaction: Withhold 1 to 2 doses; if reaction improves to
- Fourth occurrence of grade 3 dermatologic reaction: Permanently discontinue
- Grade 3 or 4 dermatologic reaction that does not recover to