- Details
- Description
-
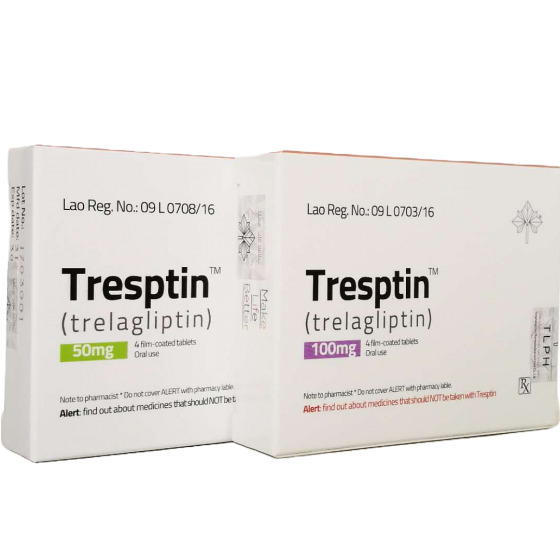
Packaging Size100mg*4Tablets
-
Strength100mg
-
CompositonTrelagliptin
-
Treatmenttype 2 diabetes
-
FormTablet
-
BrandTresptin
-
Quantity Unit4Tablets
-
ManufacturerTongmeng (Lao) Pharmaceutical & Food Co., Ltd.(TLPH)
Brand Name
Trestin, brand name: Trestin, generic name: Trelagliptin.
Indications
Type 2 diabetes
How to take the drug
1. Therapy period
Adults usually take 100mg of Trestin orally once a week.
2. Dosage form specification
● 100mg/ tablet, 4 tablets in a box;50mg/ tablet, 4 tablets in a box.
3. Contraindications
● Severe ketosis, diabetic coma or pre-coma, type 1 diabetes patients (because infusion, insulin rapid correction of hyperglycemia is necessary, so it is not suitable for use.
●Patients with severe infection, before and after surgery, and severe trauma (insulin injection to control blood sugar is not suitable for this product
●The ingredients of this product has a history of allergy patients.
Warnings and precautions
1.Use with caution for the following patients
● patients with moderate renal impairment..
● Patients currently on sulfonylureas or insulin preparations (severe hypoglycemia with other dpp-4 inhibitors has been reported)
●Pituitary or adrenal insufficiency (risk of hypoglycemia)
●Poor nutrition, hunger, irregular diet, insufficient food intake or a weak state (risk of hypoglycemia)
●Intense muscle activity (risk of hypoglycemia)
●People who drink too much (risk of hypoglycemia)
2. Important notes
●It should be noted that, because of the risk of hypoglycemia when this product is used with other diabetes drugs, the symptoms of hypoglycemia and their management should be explained in detail when used with these drugs. There is an increased risk of hypoglycemia, especially when used with sulfonylureas or insulin preparations. In order to reduce the risk of hypoglycemia in sulfonylureas or insulin preparations, the dosage of sulfonylureas or insulin preparations should be reduced when these drugs are used in combination
●This product should be taken orally once a week, and the effect will continue after the drug is stopped. The occurrence of adverse reactions related to blood glucose level should be paid enough attention to.
In addition, after the withdrawal of this product, when using other diabetes drugs, the starting time and dosage of the drug should be considered according to the blood glucose control status.
●Only for patients diagnosed with diabetes. Patients with abnormal glucose tolerance other than diabetes, positive urine glucose, etc., and diseases with similar symptoms of diabetes (renal glucuria, abnormal thyroid function, etc.) should pay attention.
●This product is only used when sufficient basic diet therapy and exercise therapy are not effective in treating diabetes.
●During the treatment of this product, in addition to regular blood glucose inspection, the drug should be closely monitored during the process. Other treatments should be considered when the effect of this product is not good for 2~3 months
●During the duration of medication, there will be situations where medication is no longer needed. In addition, when patients do not pay attention to health care, co-infection and other causes of ineffective and insufficient effect, the primary consideration should be dietary intake, blood sugar level, there is no infection, etc., should pay attention to whether to continue the usual administration, drug selection, etc.
●Because this product can cause hypoglycemia symptoms, be careful when engaging in altitude work, driving cars and other patients
●The clinical efficacy and safety of insulin preparation have not been studied
●Both glp-1 receptor agonist and glp-1 receptor agonist have hypoglycemic effect through glp-1 receptor, there is no clinical trial result of both, the efficacy and safety have not been confirmed.
Side-effect
In the domestic clinical trials at the time of approval, 103 (11.4%) of the 901 patients were observed to have adverse reactions including abnormal clinical values, mainly including hypoglycemia, nasopharyngitis, and elevated lipase.
1. Serious adverse reactions
Due to the possibility of hypoglycemia (≥0.1% and < 5%), the patient's condition should be fully observed during medication. Cases of severe hypoglycemia and loss of consciousness have been reported in combination with other dpp-4 inhibitors and sulfonylureas. Consider reducing the dose of sulfonylureas when used with sulfonylureas. In addition, sucrose is usually given when hypoglycemia occurs, while glucose should be given when hypoglycemia is observed with -glucosidase inhibitor.
2. Serious adverse reactions (similar drugs)
●Due to the possibility of acute pancreatitis, should be fully observed. In case of continuous severe abdominal pain, vomiting and other abnormalities, medication should be stopped and appropriate measures should be taken.
●Due to the possibility of intestinal obstruction, should be fully observed. In case of severe constipation, abdominal distension, continued abdominal pain, vomiting and other abnormalities, the drug should be stopped and appropriate measures should be taken.
3. Other adverse reactions
When the following adverse reactions occur, appropriate measures should be taken according to the symptoms.
| Symptoms | Value | ≥0.1% < 5 | ||
| Allergy | Rash, itching | |||
| Circulation organs | Atrial fibrillation | |||
| Liver | ALT (GPT) goes up, AST (GOT) goes up, and γ-GTP goes up | |||
| other | Increased amylase, lipase, CK (CPK), urine occult blood Yang, nasopharyngeal inflammation | |||
[How to store the drug]
Keep this medicine dark, cool and dry place..