- Details
- Description
-
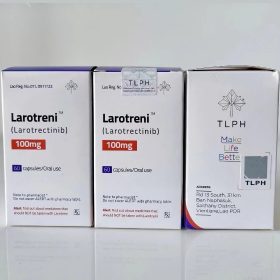
Packaging Size60c/Bottle
-
Strength25mg
-
CompositonLarotrectinib
-
TreatmentNTRK gene fusion-positive solid tumors
-
FormCapsule
-
BrandLARODX
-
Quantity Unit25mg*60c/Bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
Larotrectinib
Larotrectinib is a medication for the treatment of cancer.It is an inhibitor of tropomyosin kinase receptors TrkA, TrkB, and TrkC.
Larotrectinib was initially awarded orphan drug status in 2015, for soft tissue sarcoma, and breakthrough therapy designation in 2016 for the treatment of metastatic solid tumors with NTRK fusion.Some clinical trial results were announced in 2017. On 26 November 2018, Larotrectinib was approved by the FDA.
Larotrectinib was the first drug to be specifically developed and approved to treat any cancer containing certain mutations, as opposed to cancers of specific tissues (i.e., the approval is "tissue agnostic"). Several earlier drugs, including pembrolizumab, were eventually approved by the FDA for treatment of specific mutations independent of the type of cancer, but those drugs had been initially developed for specific cancer types.The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.
ADULT
Dosage Forms & Strengths
capsule
- 25mg
- 100mg
oral solution
- 20mg/mL
Solid Tumors
Indicated for adults and pediatric patients with solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion without a known acquired resistance mutation, are metastatic or where surgical resection is likely to result in severe morbidity, and have no alternative treatments or have progressed following treatment
100 mg PO BID
Continue until disease progression or until unacceptable toxicity
Dosage Modifications
Dosage modifications for adverse reactions
-
Body Surface Area (BSA) ≥1 m2
- First occurrence: 75 mg BID
- Second occurrence: 50 mg BID
- Third occurrence: 100 mg qDay
- Unable to tolerate 100 mg qDay: Permanently discontinue
Grade ≥3 adverse reactions
- Withhold until reaction resolves or improves to baseline or Grade ≤1
- Resolved within 4 weeks: Resume at next dosage modification
- Unresolved within 4 weeks: Permanently discontinue
Strong CYP3A4 inhibitors
- Avoid coadministration
- If unavoidable, reduce larotrectinib dose by 50%
- Once strong CYP3A4 inhibitor is discontinued for 3-5 elimination half-lives, resume larotrectinib at dose taken before initiating CYP3A4 inhibitor
Strong CYP3A4 inducers
- Avoid coadministration
- If unavoidable, double larotrectinib dose
- Once strong CYP3A4 inducer is discontinued for 3-5 elimination half-lives, resume larotrectinib at dose taken prior to initiating CYP3A4 inducer
Hepatic impairment
- Mild (Child-Pugh A): No dosage adjustment necessary
- Moderate to severe (Child-Pugh B or C): Reduce starting dose by 50%
Renal impairment
- Mild to severe: No dosage adjustment necessary
Dosing Considerations
Verify pregnancy status in females of reproductive potential before initiation
Patient selection
- Select patients based on presence of a NTRK gene fusion in tumor specimens
PEDIATRIC
Dosage Forms & Strengths
capsule
- 25mg
- 100mg
oral solution
- 20mg/mL
Solid Tumors
ndicated for adults and pediatric patients with solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion without a known acquired resistance mutation, are metastatic or where surgical resection is likely to result in severe morbidity, and have no alternative treatments or have progressed following treatment
Body surface area (BSA) <1 m2: 100 mg/m2 PO BID
BSA ≥1 m2: 100 mg PO BID
Continue until disease progression or until unacceptable toxicity
Dosage Modifications
Dosage modifications for adverse reactions
-
BSA <1 m2
- First occurrence: 75 mg/m2 BID
- Second occurrence: 50 mg/m2 BID
- Third occurrence: Not to exceed 25 mg/m2 BID; remain on 25 mg/m2 BID even if BSA becomes >1 m2 during treatment
- Unable to tolerate 25 mg/m2 BID: Permanently discontinue
BSA ≥1 m2
- First occurrence: 75 mg BID
- Second occurrence: 50 mg BID
- Third occurrence: 100 mg qDay
- Unable to tolerate 100 mg qDay: Permanently discontinue
Grade ≥3 adverse reactions
- Withhold until reaction resolves or improves to baseline or Grade ≤1
- Resolved within 4 weeks: Resume at next dosage modificatio
- Unresolved within 4 weeks: Permanently discontinue
Strong CYP3A4 inhibitors
- Avoid coadministration
- If unavoidable, reduce larotrectinib dose by 50%
- Once strong CYP3A4 inhibitor is discontinued for 3-5 elimination half-lives, resume larotrectinib at dose taken before initiating CYP3A4 inhibitor
Strong CYP3A4 inducers
- Avoid coadministration
- If unavoidable, double larotrectinib dose
- Once strong CYP3A4 inducer is discontinued for 3-5 elimination half-lives, resume larotrectinib at dose taken before initiating CYP3A4 inducer
Hepatic impairment
- Mild (Child-Pugh A): No dosage adjustment necessary
- Moderate to severe (Child-Pugh B or C): Reduce starting dose by 50%
Renal impairment
- Mild to severe: No dosage adjustment necessary
Dosing Considerations
Verify pregnancy status in females of reproductive potential before initiation
Patient selection
- Select patients based on presence of a NTRK gene fusion in tumor specimens