- Details
- Description
-
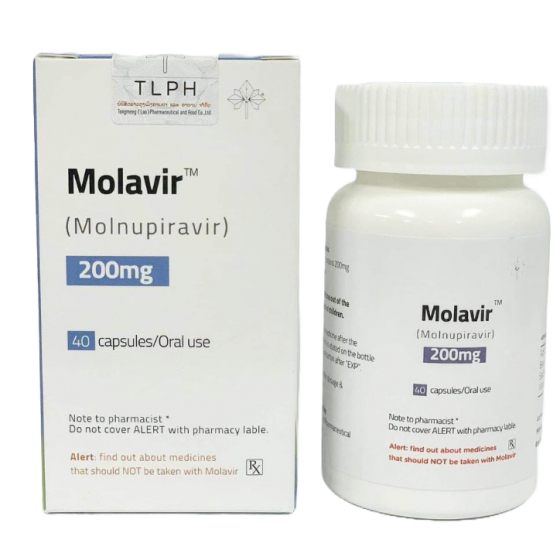
Packaging Size200mg*40Capsules/Bottle
-
Strength200mg
-
CompositonMolnupiravir
-
TreatmentTreatment COVID-19 in Adult testing positive and (SARS-CoV-2) virus
-
FormCapsule
-
BrandMolavir
-
Quantity Unit40Capsules
-
ManufacturerTongmeng (Lao) Pharmaceutical & Food Co., Ltd.(TLPH)
Molavir (Molnupiravir)200mg
Dosage Forms : Capsule
Strengths: 200mg
COVID-19 Disease Treatment
Treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults testing positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, and who are at high risk for progression to severe COVID-19, including hospitalization or death
800 mg PO q12hr for 5 days.
Initiate as soon as possible after COVID-19 diagnosis and within 5 days of symptom onset
Completing the full 5-day treatment course and continuing to isolate in accordance with public health recommendations are important to maximize viral clearance and minimize viral transmission
If patient is hospitalized due to severe or critical COVID-19 after starting treatment with Molnupiravir, completion of the full 5-day treatment is at the healthcare provider’s discretion
Dosage Modifications
Renal impairment
- Mild or moderate (eGFR >30 mL/min): No dosage adjustment necessary
- Severe (eGFR <30 mL/min), end-stage renal disease, or patients on dialysis: Pharmacokinetics not evaluated; not expected to have a significant effect on NHC (N4-hydroxycytidine) exposure
Hepatic impairment
- Mild to severe (Child-Pugh Class A to C): No dosage adjustment required
- Preclinical data indicate that hepatic elimination is not expected to be a major route of NHC elimination
Dosing Considerations
Verify pregnancy status in females of childbearing potential before initiating
Limitations of use
- Not authorized for use in patients aged <18 years
- Not authorized for initiation of treatment in patients requiring hospitalization owing to COVID-19
- Not authorized for use for >5 consecutive days
- Not authorized for preexposure or postexposure prophylaxis for prevention of COVID-19.
Adverse Effects
1-10%
Diarrhea (2%)
Nausea (1%)
Dizziness (1%)
≤2%
- Selected Grade 3 and 4 laboratory abnormalities in chemistry (ALT, AST, creatinine, and lipase) and hematology (hemoglobin, platelets, and leukocytes)
Required reporting for serious adverse events and medication errors
Prescribers and/or the provider’s designee are/is responsible for mandatory reporting of all serious adverse events and medication errors potentially drug-related within 7 calendar days from the onset of the event
Serious adverse events are defined as
- Death or a life-threatening adverse event;
- A medical or surgical intervention to prevent death, a life-threatening event, hospitalization, disability, or congenital anomaly;
- Inpatient hospitalization or prolongation of existing hospitalization;
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions; or
- A congenital anomaly/birth defect
Cautions
There are limited clinical data available; serious and unexpected adverse events may occur that have yet to be reported
May cause fetal harm when administered to pregnant females
Bone and cartilage toxicity
- Not authorized for use in patients aged <18 years owing to possible effects on bone and cartilage growth
- Bone and cartilage toxicity was observed in rats after repeated dosing
- Safety and efficacy have not been established in pediatric patients
Missed dose
- <10 hours: Take missed dose as soon as possible, and resume normal dosing schedule
- >10 hours: Skip dose, and take the next dose at the regularly scheduled time
- Do not double dose to make up for missed dose
Storage
Store at 20-25ºC (68-77ºF); excursions permitted to 15-30ºC (59-86ºF)