- Details
- Description
-
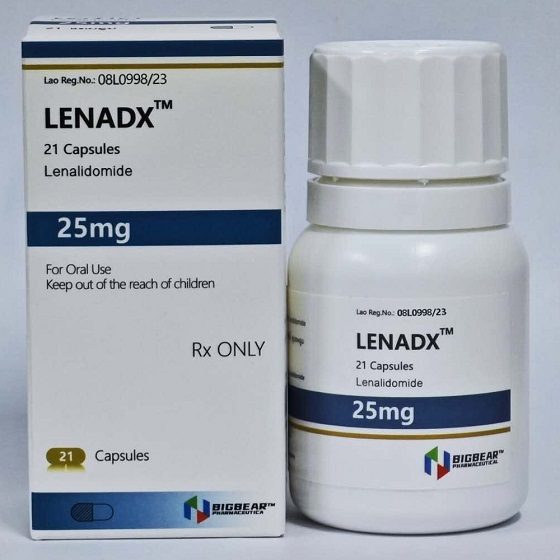
Packaging Size21c/bottle
-
Strength25mg
-
CompositonLenalidomide
-
TreatmentMyeloma,Myelodysplastic Syndromes
-
FormCapsule
-
BrandLENADX
-
Quantity Unit25mg*21c/bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
Lenalidomide is a medication used to treat multiple myeloma, smoldering myeloma, and myelodysplastic syndromes (MDS). For multiple myeloma, it is used after at least one other treatment and generally with dexamethasone. It is taken by mouth.
Common side effects include diarrhea, itchiness, joint pain, fever, headache, and trouble sleeping. Severe side effects include low blood platelets, low white blood cells, and blood clots. Use during pregnancy may harm the fetus. The dose may need to be adjusted in people with kidney problems. It has a chemical structure similar to thalidomide but has a different mechanism of action. How it works is not entirely clear as of 2019.
Lenalidomide was approved for medical use in the United States in 2005. It is on the World Health Organization's List of Essential Medicines.
Medical uses
Multiple myeloma
Lenalidomide is used to treat multiple myeloma. It is a more potent molecular analog of thalidomide, which inhibits tumor angiogenesis, tumor-secreted cytokines, and tumor proliferation through induction of apoptosis.
Lenalidomide is effective at inducing a complete or "very good partial" response and improves progression-free survival. Adverse events more common in people receiving lenalidomide for myeloma include neutropenia, deep vein thrombosis, infections, and an increased risk of other hematological malignancies. The risk of second primary hematological malignancies does not outweigh the benefit of using lenalidomide in relapsed or refractory multiple myeloma. It may be more difficult to mobilize stem cells for autograft in people who have received lenalidomide.
In 2006, lenalidomide received U.S. Food and Drug Administration (FDA) clearance for use in combination with dexamethasone in people with multiple myeloma who have received at least one prior therapy. In 2017, the FDA approved lenalidomide as standalone maintenance therapy (without dexamethasone) for people with multiple myeloma following autologous stem cell transplant.
In 2009, The National Institute for Health and Clinical Excellence issued a final appraisal determination approving lenalidomide in combination with dexamethasone as an option to treat people with multiple myeloma who have received two or more prior therapies in England and Wales.
The use of lenalidomide combined with other drugs was evaluated. It was seen that the drug combinations of lenalidomide plus dexamethasone and continuous bortezomib plus lenalidomide plus dexamethasone probably result in an increase of the overall survival.
Myelodysplastic syndromes
Lenalidomide was approved by the FDA on 27 December 2005 for patients with low- or intermediate-1-risk myelodysplastic syndromes who have chromosome 5q deletion syndrome (5q- syndrome) with or without additional cytogenetic abnormalities. It was approved on 17 June 2013 by the European Medicines Agency for use in patients with low- or intermediate-1-risk myelodysplastic syndromes who have 5q- deletion syndrome but no other cytogenetic abnormalities and are dependent on red blood cell transfusions, for whom other treatment options have been found to be insufficient or inadequate.
Mantle cell lymphoma
Lenalidomide is approved by FDA as a specialty drug requiring a specialty pharmacy distribution for mantle cell lymphoma in patients whose disease has relapsed or progressed after at least two prior therapies, one of which must have included the medicine bortezomib.
AL amyloidosis
Although not specifically approved by the FDA for use in treating AL amyloidosis, lenalidomide is widely used in the treatment of that condition, often in combination with dexamethasone.
Why is this medication prescribed?
Lenalidomide is used to treat a certain type of myelodysplastic syndrome (a group of conditions in which the bone marrow produces blood cells that are misshapen and does not produce enough healthy blood cells). Lenalidomide is also used along with dexamethasone to treat people with multiple myeloma (a type of cancer of the bone marrow). It is also used to treat people with multiple myeloma after a hematopoietic stem-cell transplant (HSCT; procedure in which certain blood cells are removed from the body and then returned to the body). Lenalidomide is also used to treat people with mantle cell lymphoma (a fast-growing cancer that begins in the cells of the immune system) who have been treated with bortezomib (Velcade) and at least one other medication. Lenalidomide should not be used to treat people with chronic lymphocytic leukemia (a type of cancer of the white blood cells that gets worse slowly over time) unless they are participating in a clinical trial (research study to see whether a medication may be used safely and effectively to treat a certain condition). Lenalidomide is in a class of medications called immunomodulatory agents. It works by helping the bone marrow to produce normal blood cells and by killing abnormal cells in the bone marrow.
How should this medicine be used?
Lenalidomide comes as a capsule to take by mouth. When lenalidomide is used to treat myelodysplastic syndrome, it is usually taken with or without food once daily. When lenalidomide is used to treat multiple myeloma or mantle cell lymphoma, it is usually taken with or without food once daily for the first 21 days of a 28-day cycle. When lenalidomide is used to treat multiple myeloma after HSCT, it is usually taken with or without food once daily for 28 days of a 28-day cycle. The 28-day cycle regimen may be repeated as recommended by your doctor based on your body's response to this medication. Take lenalidomide at around the same time of day every day that you take it. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take lenalidomide exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Swallow the capsules whole with plenty of water; do not break, chew, or open them. Handle the capsules as little as possible. If you touch a broken lenalidomide capsule or the medicine in the capsule, wash that area of your body with soap and water. If the medicine in the capsule gets into your mouth, nose, or eyes, wash it away with plenty of water.
Your doctor may need to interrupt your treatment or reduce your dose if you experience certain side effects. Be sure to tell your doctor how you are feeling during your treatment with lenalidomide.
Other uses for this medicine
This medication may be prescribed for other uses; ask your doctor or pharmacist for more information.