- Details
- Description
-
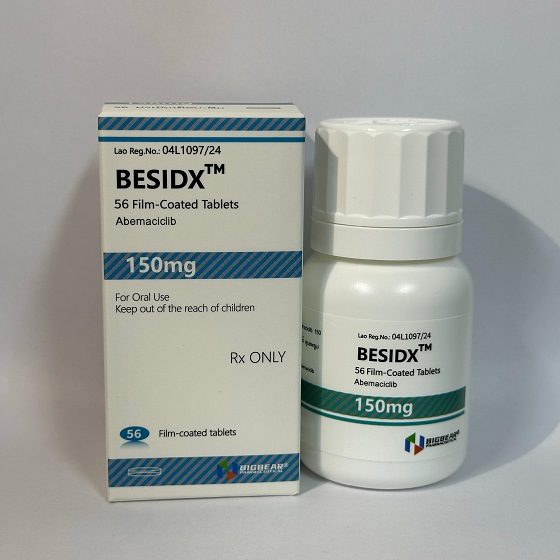
Packaging Size56T/Bottle
-
Strength150mg
-
CompositonAbemaciclib
-
TreatmentHR-positive & HER2-negative breast cancer
-
FormTablet
-
BrandBESIDX
-
Quantity Unit150mg*56T/Bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
Abemaciclib is used in combination with an aromatase inhibitor such as anastrozole (Arimidex), exemestane (Aromasin), or letrozole (Femara) to treat a certain type of hormone receptor-positive, early breast cancer. Abemaciclib is also used along with fulvestrant (Faslodex) to treat a certain type of hormone receptor-positive, advanced breast cancer (breast cancer that depends on hormones such as estrogen to grow) or breast cancer that has spread to other parts of the body after treatment with an antiestrogen medication such as tamoxifen. Abemaciclib is also used along with anastrozole (Arimidex), exemestane (Aromasin), or letrozole (Femara) as a first treatment of hormone receptor-positive, advanced breast cancer or breast cancer that has spread to other parts of the body. Abemaciclib is also used alone to treat a certain type of hormone receptor-positive, advanced breast cancer or breast cancer that has spread to other parts of the body in people who have already been treated with an antiestrogen medication and chemotherapy. Abemaciclib is in a class of medications called kinase inhibitors. It works by blocking the action of an abnormal protein that signals cancer cells to multiply. This helps slow or stop the spread of cancer cells.
Early Breast Cancer
Indicated in combination with endocrine therapy (tamoxifen or an aromatase inhibitor) for adjuvant treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, nodepositive, early breast cancer at high risk of recurrence
150 mg PO BID PLUS tamoxifen or an aromatase inhibitor (see Prescribing Information)
Continue for 2 years, or until disease recurrence or unacceptable toxicity
Advanced or Metastatic Breast Cancer
Monotherapy
- Indicated as monotherapy for adults with HR-positive, HER2-negative advanced or metastatic breast cancer with disease progression following endocrine therapy and prior chemotherapy in metastatic setting
- 200 mg PO BID
- Continue until disease progression or unacceptable toxicity
Combination therapy with aromatase inhibitor
- Indicated in combination with an aromatase inhibitor as initial endocrine-based therapy for HR-positive, HER2-negative advanced or metastatic breast cancer
- 150 mg PO BID PLUS an aromatase inhibitor (see Prescribing Information)
- Continue until disease progression or unacceptable toxicity
Combination therapy with fulvestrant
- Indicated, in combination with fulvestrant, for adults with HR-positive, HER2-negative advanced or metastatic breast cancer with disease progression following endocrine therapy
- 150 mg PO BID PLUS
- Fulvestrant 500 mg IM on Days 1, 15, and 29, and then once monthly thereafter
- Continue until disease progression or unacceptable toxicity
Dosage Modifications
Dosage modifications for adverse effects
-
Combined with fulvestrant, tamoxifen, or aromatase inhibitor
- Starting dose: 150 mg BID
- First dose reduction: 100 mg BID
- Second dose reduction: 50 mg BID
- Discontinue if unable to tolerate 50 mg BID
-
Monotherapy
- Starting dose: 200 mg BID
- First dose reduction: 150 mg BID
- Second dose reduction: 100 mg BID
- Third dose reduction: 50 mg BID
- Discontinue if unable to tolerate 50 mg BID