- Details
- Description
-
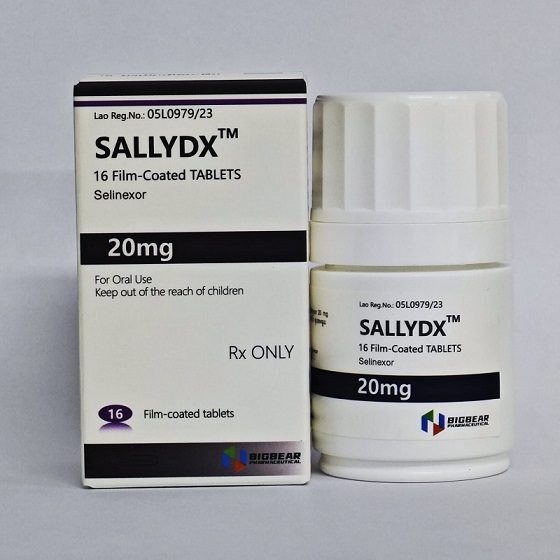
Packaging Size16T/bottle
-
Strength20mg
-
CompositonSelinexor
-
TreatmentDiffuse Large B-Cell Lymphoma,Multiple Myeloma
-
FormTablet
-
BrandSALLYDX
-
Quantity Unit20mg*16T/bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
Selinexor is used along with dexamethasone to treat multiple myeloma (a type of cancer of the bone marrow) that has returned or that did not respond to at least 4 other treatments.
Multiple Myeloma
In combination with bortezomib and dexamethasone (SVd)
- Indicated, in combination with bortezomib and dexamethasone, for multiple myeloma (MM) have received ≥1 prior therapy
-
35-day cycle
- Selinexor 100 mg PO qWeek on Day 1; continue until disease progression or unacceptable toxicity
- Bortezomib 1.3 mg/m2 SC qWeek on Day of each week x 4 weeks followed by 1 week off
- Dexamethasone 20 mg PO twice weekly on Days 1 and 2 of each week
- Refer to prescribing information of bortezomib and dexamethasone for additional dosing information
In combination with dexamethasone (Sd)
- Indicated in combination with dexamethasone for adults with relapsed or refractory multiple myeloma (RRMM) who have received at least 4 prior therapies and whose disease is refractory to at least 2 proteasome inhibitors, at least 2 immunomodulatory agents, and an anti-CD38 monoclonal antibody
- Selinexor 80 mg PO plus dexamethasone 20 mg PO on Days 1 and 3 of each week
- Continue until disease progression or unacceptable toxicity
- Refer to prescribing information of dexamethasone for additional dosing information
Diffuse Large B-Cell Lymphoma
Indicated for relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, in patients previously treated with at least 2 lines of systemic therapy
60 mg PO on Days 1 and 3 of each week
Continue until disease progression or unacceptable toxicity
Dosage Modifications
Dosage reduction recommendations for adverse effects
-
SVd
- Starting dose: 100 mg once weekly
- First reduction: 80 mg once weekly
- Second reduction: 60 mg once weekly
- Third reduction: 40 mg once weekly
- Fourth reduction: Permanently discontinue
-
Sd
- Starting dose: 80 mg on Days 1 and 3 each week (160 mg/week)
- First reduction: 100 mg once weekly
- Second reduction: 80 mg once weekly
- Third reduction: 60 mg once weekly
- Fourth reduction: Permanently discontinue
-
DLBCL
- Starting dose: 60 mg on Days 1 and 3 each week (120 mg/week)
- First reduction: 40 mg Days 1 and 3 of each week (80 mg total per week)
- Second reduction: 60 mg once weekly
- Third reduction: 40 mg once weekly
- Fourth reduction: Permanently discontinue