- Details
- Description
-
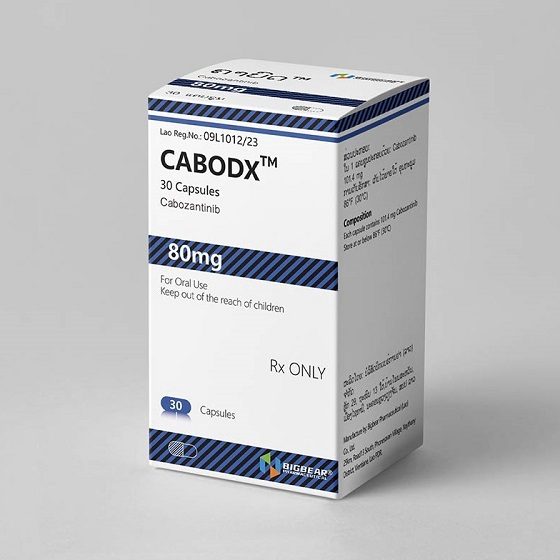
Packaging Size30C/bottle
-
Strength80mg
-
CompositonCabozantinib
-
TreatmentMedullary Thyroid Cancer(MTC)
-
FormCapsule
-
BrandCABODX
-
Quantity Unit80mg*30C/bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
Cabozantinib is an anti-cancer medication used to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma. It is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and also inhibits AXL and RET.
Medullary Thyroid Cancer
Capsule only
Indicated for treatment of progressive, metastatic medullary thyroid cancer (MTC)
140 mg PO qDay
Continue until disease progression or unacceptable toxicity occurs
Renal Cell Carcinoma
Tablet only
Combination with nivolumab
- Indicated in combination with nivolumab for first-line treatment of advanced renal cell carcinoma (RCC)
- Cabozantinib 40 mg PO qDay PLUS nivolumab 240 mg IV q2Weeks or 480 mg IV q4Weeks
- Continue until disease progression or unacceptable toxicity
- Nivolumab only: Continue for up to 2 years
Single agent
- Indicated for advanced RC
- 60 mg PO qDay
- Continue until disease progression or unacceptable toxicity occurs
Hepatocellular Carcinoma
Tablet only
Indicated for patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib
60 mg PO qDay
Continue until disease progression or unacceptable toxicity occurs
Differentiated Thyroid Cancer
Indicated for locally advanced or metastatic differentiated thyroid cancer (DTC) in adults who have progressed following prior VEGFR-targeted therapy and who are radioactive iodine-refractory or ineligible
BSA >1.2 m2: 60 mg PO qDay
Continue until disease progression or unacceptable toxicity
Dosage Modifications
Dosage reductions (Cabometyx)
-
Monotherapy with BSA >1.2 m2
- First dose reduction: 40 mg PO qDay
- Second dose reduction: 20 mg PO qDay
- Previously receiving 20 mg qDay: Resume at same dose
- Unable to tolerate 20 mg qDay: Discontinue treatment
-
In combination with nivolumab
- First dose reduction: 20 mg PO qDay
- Second dose reduction: 20 mg PO every other day
- Previously receiving 20 mg every other day: Resume at same dose
- Unable to tolerate 20 mg every other day: Discontinue treatment
Withhold dose until Grade ≤1 or until complete resolution
- Resume at reduced dose
- Grade ≥2 diarrhea
- Grade 3 or intolerable grade 2 palmar-plantar erythrodysesthesia
- Grade 2 or 3 proteinuria
- Any grade osteonecrosis of jaw (ONJ)
- Grade ≥3 or intolerable grade 2 other adverse reactions
Withhold dose until Grade ≤1
-
Tablet
- Consider corticosteroid therapy if withheld or discontinued when administered in combination with nivolumab
- ALT or AST >3x ULN but ≤10x ULN with concurrent total bilirubin [TB] <2x ULN
- After recovery, consider rechallenge with one or both of Cabometyx and nivolumab
- If rechallenging with nivolumab with or without Cabometyx, refer to nivolumab prescribing information
Withhold dose until adequately controlled at Grade ≤2
- Resume at reduced dose
- Grade 3 hypertension and hypertensive crisis
Permanently discontinue
- Grades 3 or 4 hemorrhage
- Any grade gastrointestinal perforation
- Grade 4 fistula
- Any grade acute myocardial infarction
- Grade ≥2 cerebral infarction
- Grade 3 or 4 arterial thromboembolic events
- Grade 4 venous thromboembolic events
- Grade 4 hypertension or uncontrolled hypertensive crisis
- Nephrotic syndrome
- Reversible posterior leukoencephalopathy syndrome (RPLS)
- ALT or AST >10x ULN or >3x ULN with concurrent TB ≥2x ULN; permanently discontinue both nivolumab and Cabometyx
CYP3A4 inhibitors
- Avoid coadministration with strong CYP3A4 inhibitors
- Cometriq: If strong CYP3A4 inhibitor required, decrease cabozantinib dose by 40 mg/day (eg, from 140 mg to 100 mg qDay)
- Cabometyx: If strong CYP3A4 inhibitor required, decrease cabozantinib dose by 20 mg/day (eg, from 60 mg to 40 mg qDay)
- Resume previous dose 2-3 days after strong CYP3A4 inhibitor discontinued
CYP3A4 inducers
- Avoid coadministration of strong CYP3A4 inducers
- Cometriq: If strong CYP3A4 inducer required, increase dose by 40 mg/day (eg, from 140 mg to 180 mg qDay); do not exceed 180 mg/day
- Cabometyx: If strong CYP3A4 inducer required, increase dose by 20 mg/day (eg, from 60 mg to 80 mg qDay); do not exceed 80 mg/day
- Resume previous dose 2-3 days after strong CYP3A4 inducer discontinued
Hepatic impairment
-
Tablet
- Mild-to-moderate (Child-Pugh A or B): Reduce starting dose to 80 mg/day
- Moderate (Child-Pugh C): Not recommended
-
Tablet
- Mild (Child-Pugh A): No dosage adjustment necessary
- Moderate (Child-Pugh B): Reduce starting dose to 40 mg/day
- Severe (Child-Pugh C): Avoid use
Renal impairment
- Mild-to-moderate (CrCl ≥30 mL/min): No dosage adjustment necessary
- Severe (CrCl <30 mL/min): No experience