- Details
- Description
-
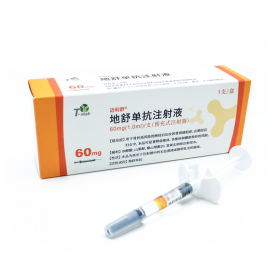
Packaging Size1vial/Box
-
Strength20mg/1ml
-
CompositonEfbemalenograstim alfa (艾贝格司亭-α)
-
Treatmentchemotherapy-induced neutropenia (CIN)
-
FormInjection
-
BrandRyzneuta (亿立舒)
-
Quantity Unit20mg/1ml*1vail/Box
-
ManufacturerEvive Biotech,china
Ryzneuta® (Efbemalenograstim alfa-vuxw), sold under the brand name 亿立舒® in china, is a leukocyte growth factor used for reducing the incidence of infection, as manifested by febrile neutropenia (FN), in adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer medicines associated with a clinically significant incidence of febrile neutropenia (FN).
It was approved for medical use in China in May 2023, and the United States in November 2023.
November 16, 2023 – The FDA approved Evive Biotechnology’s Ryzneuta (efbemalenograstim alfa-vuxw), to decrease the incidence of infection, as manifested by febrile neutropenia, in adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia.