- Details
- Description
-
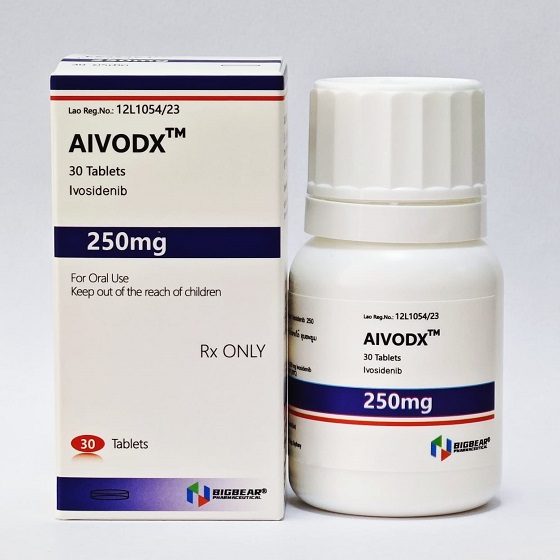
Packaging Size30t/bottle
-
Strength250mg
-
CompositonIvosidenib
-
TreatmentAdult patients with an IDH1 mutation with:newly-diagnosed acute myeloid leukemia (AML) or cholangiocarcinoma (bile duct cancer)
-
FormTablet
-
BrandAIVODX
-
Quantity Unit30T/bottle/box
-
ManufacturerBIGBEAR Pharma,LAOs PDR
Ivosidenib is a first-in-class isocitrate dehydrogenase-1 (IDH1) inhibitor. Ivosidenib is an anti-cancer medication for the treatment of acute myeloid leukemia (AML) and cholangiocarcinoma.
Acute Myeloid Leukemia
Newly-diagnosed acute myeloid leukemia (AML)
- Indicated in combination with azacitidine or as monotherapy for newly-diagnosed acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation in adults aged ≥75 years, or who have comorbidities that preclude use of intensive induction chemotherapy
-
Combination regimen
- 500 mg PO qDay until disease progression or unacceptable toxicity
- For patients without disease progression or unacceptable toxicity, continue for minimum of 6 months to allow time for clinical response
- Start ivosidenib on Cycle 1 Day 1 in combination with azacitidine 75 mg/m2 SC/IV qDay on Days 1-7 (or Days 1-5 and 8-9) of each 28-day cycle
-
Monotherapy
- 500 mg PO qDay until disease progression or unacceptable toxicity
- For patients without disease progression or unacceptable toxicity, continue for minimum of 6 months to allow time for clinical response
Relapsed or refractory AML
- Indicated for patients with relapsed or refractory AML with a susceptible IDH1 mutation as detected by an FDA-approved test
- 500 mg PO qDay
- Continue until disease progression or unacceptable toxicity; treat for a minimum of 6 months to allow time for clinical response
Cholangiocarcinoma
Indicated for locally advanced or metastatic cholangiocarcinoma in previously treated adults with IDH1 mutation
500 mg PO qDay
Continue until disease progression or unacceptable toxicity