- Details
- Description
-
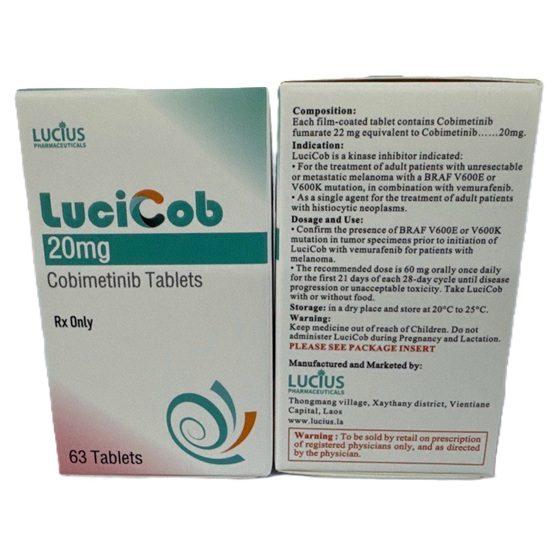
Packaging Size63t/bottle
-
Strength20mg
-
CompositonCobimetinib
-
TreatmentBRAF V600E or V600K mutation Melanoma & Histiocytic Neoplasms
-
FormTablet
-
BrandLuciCob
-
Quantity Unit20mg*63t/Box
-
ManufacturerLucius Pharmaceuticals (Lao) Co.,Ltd
About Cobimetinib
Cobimetinib is a MEK inhibitor. Cobimetinib is an anti-cancer medication used to treat melanoma and histiocytic neoplasms.
Melanoma
Indicated for unresectable or metastatic melanoma in patients with a BRAF V600E or V600K mutation, in combination with vemurafenib
60 mg PO qDay for the first 21 days of each 28-day cycle until disease progression or unacceptable toxicity
Vemurafenib: 960 mg PO BID on days 1-28 of an every 28-day cycle
Histiocytic Neoplasms
Indicated as a single agent for adults with histiocytic neoplasms
60 mg PO qDay for the first 21 days of each 28-day cycle until disease progression or unacceptable toxicity
Dosage Modifications
New primary malignancies (cutaneous and noncutaneous): No dosage modification required
Avoid concurrent use of cobimetinib and strong or moderate CYP3A inducers including but not limited to carbamazepine, efavirenz, phenytoin, rifampin, and St. John’s Wort
Hepatic impairment
- Mild-to-severe (Child-Pugh A to C): No dosage adjustment necessary
Renal impairment
- Mild-to-moderate (CrCl 30-89 mL/min): No dosage adjustment necessary
- Severe (CrCl <30 mL/min): Safety and efficacy not established
Coadministration with CYP3A inhibitors
- Do not take strong or moderate CYP3A inhibitors while taking cobimetinib
- If concurrent short-term (≤14 days) use of moderate CYP3A inhibitors is unavoidable for patients taking cobimetinib 60 mg, reduce dose to 20 mg; after the moderate CYP3A inhibitor is discontinued, resume previous cobimetinib dose
- Use an alternative to a strong or moderate CYP3A inhibitor in patients who are taking a reduced dose of cobimetinib (40 or 20 mg daily)
Dose reductions
- First dose reduction: 40 mg PO qDay
- Second dose reduction: 20 mg PO qDay
- Subsequent modification: Permanently discontinue cobimetinib if unable to tolerate 20-mg dose
Hemorrhage
- Grade 3: Withhold cobimetinib for up to 4 wk; if improved to grade 0 or 1, resume at the next lower dose level; permanently discontinue if not improved within 4 wk
- Grade 4: Permanently discontinue
Cardiomyopathy
-
Asymptomatic
- Definition: Absolute decrease in LVEF from baseline of >10% and less than institutional lower limit of normal (LLN) Withhold cobimetinib for 2 wk; repeat LVEF
- Resume at next lower dose if all of the following are present: LVEF is ≥LLN and absolute decrease from baseline LVEF is ≤10%
- Permanently discontinue if any of the following are present: LVEF is 10%
-
Symptomatic LVEF decrease from baseline
- Withhold cobimetinib for 4 wk; repeat LVEF
- Resume at next lower dose if all of the following are present:
- Symptoms resolve and LVEF is ≥LLN and absolute decrease from baseline LVEF is ≤10%
- Permanently discontinue if any of the following are present:
- Symptoms persist, or LVEF is 10%
Dermatologic reactions
- Grade 2 (intolerable) or grades 3 or 4: Withhold or reduce dose
Retinopathy or retinal vein occlusion
- Serous retinopathy: Withhold for up to 4 wk; if signs and symptoms improve, resume at the next lower dose level; permanently discontinue if not improved or symptoms recur at the lower dose within 4 wk
- Retinal vein occlusion: Permanently discontinue
Hepatoxicity
- First occurrence grade 4: Withhold for up to 4 wk; if improved to grade 0 or 1, resume at the next lower dose level; permanently discontinue if not improved within 4 wk
- Recurrent grade 4: Permanently discontinue
Rhabdomyolysis and elevated CPK
- Grade 4 creatine phosphokinae (CPK) elevation or any CPK elevation plus myalgia: Withhold for up to 4 wk; if improved to ≤grade 3, resume at the next lower dose level; permanently discontinue if not improved within 4 wk
Photosensitivity
- Grade 2 (intolerable), grades 3 or 4: Withhold for up to 4 wk; if improved to grade 0 or 1, resume at the next lower dose level; permanently discontinue if not improved within 4 wk
Other adverse events
-
Grade 2 (intolerable) adverse reactions or any grade 3
- Withhold for up to 4 wk; if improved to grade 0 or 1, resume at the next lower dose level; permanently discontinue if not improved within 4 wk
-
First occurrence of any grade 4 adverse reaction
- Withhold until adverse reaction improves to grade 0 or 1 and then resume at the next lower dose level, OR
- Permanently discontinue
-
Recurrent grade 4 adverse reaction
- Permanently discontinue