- Details
- Description
-
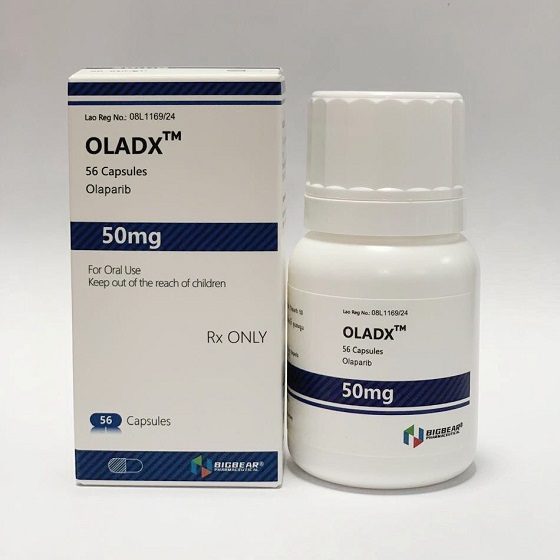
Packaging Size56c/bottle
-
Strength50mg
-
CompositonOlaparib
-
TreatmentOvarian cancer, fallopian tube cancer, peritoneal cancer, pancreatic cancer, and prostate cancer.
-
FormCapsule
-
BrandOLADX
-
Quantity Unit50mg*56c/bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
About Olaparib
Olaparib is a poly (ADP ribose) polymerase (PARP) inhibitor, at least before monotherapy Germline BRCA gene mutations associated with harmful or suspected harmful after 3 chemotherapy (FDA also approved the gene Detection reagent) for patients with advanced ovarian cancer.
Olaparib was approved by the FDA due to its objective response rate and response duration. Further of this indication
Approval depends on the results of clinical benefit in confirmatory clinical trials.
Dose and administration method
- Method of administration
- The recommended dose is 400 mg twice a day, orally, either before or after meals.
- Continue to use until the disease progresses or unacceptable toxicity occurs.
- If adverse reactions occur, consider discontinuing the medication or reducing the dose.
- For patients with mild renal impairment (creatinine clearance (CLcr)=31-50mL/min), the dose is reduced 300mg/time.
- Dosage specifications
- Capsule, 50mg.
- Contraindications: None.
Warnings and Precautions
- Myelodysplastic syndrome/acute myelogenous leukemia (MDS/AML): The patient is taking European MDS/AML appeared after Lini, and a large number of reports were fatal. Baseline monitoring of whole blood cells every month after treatment Cell count. Once diagnosed with MDS/AML, Orini was stopped immediately.
- Pneumonia: The patient develops pneumonia after taking Olini. In some cases, it is fatal. Once the lungs appear The treatment was discontinued for inflammatory symptoms, and Olini was discontinued after diagnosis.
- Fetal-embryotoxicity: Olini can be fatal to the fetus. Advise women with reproductive capacity to suffer Patients take effective contraceptive measures during treatment.
Adverse reactions
- Common adverse reactions (≥20%) in clinical trials include anemia, nausea, fatigue (including weakness), Vomiting, diarrhea, dizziness, indigestion, headache, loss of appetite, nasopharyngitis/pharyngitis/URI, cough, close Joint pain/muscle pain, back pain, dermatitis/rash, abdominal pain/discomfort.
- Common laboratory abnormalities (≥25%) include: creatinine, increase in average red blood cell volume; blood Globin, lymphocytes, absolute neutrophils, thrombocytopenia.
Storage method
Store between 20-25°C.