- Details
- Description
-
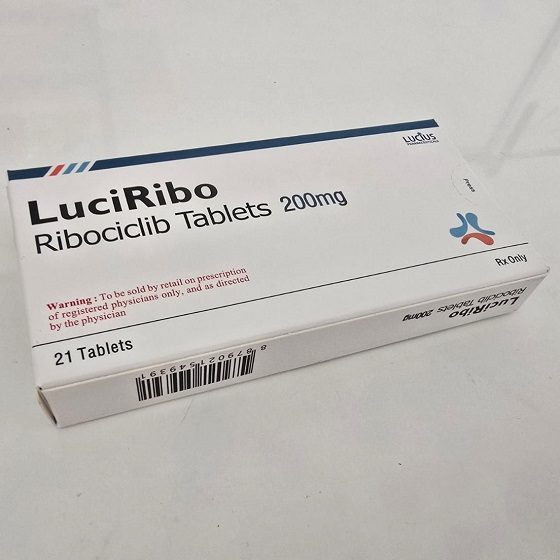
Packaging Size21t/Box
-
Strength200mg
-
CompositonRibociclib
-
TreatmentHER2 (-) negative breast cancer
-
FormTablet
-
BrandLuciRibo
-
Quantity Unit200mg*21t/Box
-
ManufacturerLucius Pharmaceuticals (Lao) Co.,Ltd
About Ribociclib
Ribociclib is a targets proteins called cyclin dependant kinase 4 and cyclin dependant 6 (CDK 4 and CDK 6) on breast cancer cells. CDK 4 and CDK 6 are proteins that stimulate cancer cells to divide and grow. Ribociclib works by blocking these proteins.
Breast Cancer
Early breast cancer
- Indicated in combination with an aromatase inhibitor for the adjuvant treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative stage II and III early breast cancer at high risk of recurrence
- 400 mg PO daily for 21 consecutive days followed by 7 days off treatment
- Repeat in 28-day cycles for 3 years or until disease recurrence or unacceptable toxicity
- Refer to prescribing information for the recommended dose of the aromatase inhibitor
Advanced or metastatic breast cancer
- Indicated for HR-positive, HER2-negative advanced or metastatic breast cancer
-
In combination with an aromatase inhibitor as initial endocrine-based therapy
- 600 mg PO daily for 21 consecutive days followed by 7 days off treatment
- Repeat in 28-day cycles until disease recurrence or unacceptable toxicity
- Refer to prescribing information for the recommended dose of the aromatase inhibitor
-
In combination with fulvestrant as initial endocrine-based therapy or following disease progression on endocrine therapy
- 600 mg PO daily for 21 consecutive days followed by 7 days off treatment; repeat in 28-day cycles until disease recurrence or unacceptable toxicity, PLUS
- Fulvestrant 500 mg IM on Days 1,15, and 29, followed by 500 mg IM monthly thereafter
Dosage Modifications
Review the prescribing information for the coadministered aromatase inhibitor or fulvestrant for dosage modifications
Recommended dose modifications for adverse effects
-
Early breast cancer
- Starting dose: 400 mg/day
- Dose reduction: 200 mg/day; discontinue if further dose adjustment is required
-
Advanced or metastatic breast cancer
- Starting dose: 600 mg/day
- First dose reduction: 400 mg/day
- Second dose reduction: 200 mg/day; discontinue if further dose adjustment is required