- Details
- Description
-
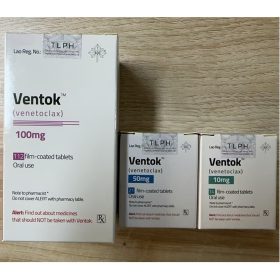
Packaging Size112T/bottle
-
Strength100mg
-
CompositonVenetoclax
-
TreatmentChronic lymphocytic leukemia(CLL), Small lymphocytic lymphoma(SLL), Acute myeloid leukemia(AML)
-
FormTablets
-
BrandVECLADX 100
-
Quantity Unit100mg*112T/Bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
Venetoclax is a medication used to treat adults with chronic lymphocytic leukemia(CLL), small lymphocytic lymphoma(SLL), or acute myeloid leukemia(AML).
Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma
Indicated for patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL)
Dose ramp-up phase
- Administer dose according to a weekly ramp-up schedule over 5 weeks to the recommended daily dose of 400 mg
- Ramp-up dosing schedule designed to gradually reduce tumor burden and decrease risk of tumor lysis syndrome
- Week 1: 20 mg PO qDay
- Week 2: 50 mg PO qDay
- Week 3: 100 mg PO qDay
- Week 4: 200 mg PO qDay
- Week 5 and beyond: 400 mg PO qDay
Monotherapy
- 400 mg PO qDay after completing the 5-week dose ramp-up schedule
- Continue until disease progression or unacceptable toxicity
Combination with obinutuzumab
-
Cycle 1
- Day 1: Obinutuzumab 100 mg IV
- Day 2: Obinutuzumab 900 mg IV
- Days 8 and 15: Obinutuzumab 1000 mg IV
- Day 22: Start venetoclax according to 5-week ramp up schedule
-
Cycle 2
- Day 1: Obinutuzumab 1000 mg IV
- Day 28: After completing the ramp-up phase on Cycle 2 Day 28, continue venetoclax 400 mg qDay from Cycle 3 Day 1 until Cycle 12 Day 28
-
Cycles 3-6
- Day 1: Obinutuzumab 1000 mg IV
- Days 1-28: Continue venetoclax 400 mg PO qDay
-
Cycles 7-12
- Days 1-28: Continue venetoclax 400 mg PO qDay
Combination with rituximab
-
Venetoclax
- Complete 5-week ramp-up dosing to reach 400 mg PO qDay
- Continue venetoclax 400 mg PO qDay for 24 months from Cycle 1 Day 1 of rituximab
-
Rituximab
- Initiate 375 mg/m² IV after patient has received venetoclax 400 mg/day x 7 days (ie, this will be Day 1 of Cycle 1)
- 500 mg/m² IV on Day 1 for Cycles 2-6
Acute Myeloid Leukemia
Indicated in combination with azacitidine or decitabine or low-dose cytarabine for treatment in adults (≥75 years) who are newly-diagnosed acute myeloid leukemia (AML) or who have comorbidities that preclude use of intensive induction chemotherapy
Venetoclax dosing depends on the combination agent
Dose ramp-up phase
- Day 1: 100 mg PO qDay
- Day 2: 200 mg PO qDay
- Day 3: 400 mg PO qDay
- Day 4 and beyond (in combination with decitabine or azacitidine): 400 mg PO qDay
- Day 4 and beyond (in combination with low-dose cytarabine): 600 mg PO qDay
- In combination with decitabine, azacitidine, or low-dose cytarabine: Continue until disease progression or unacceptable toxicity
Refer to prescribing information for azacitidine, decitabine, or cytarabine for additionaldosing information