- Details
- Description
-
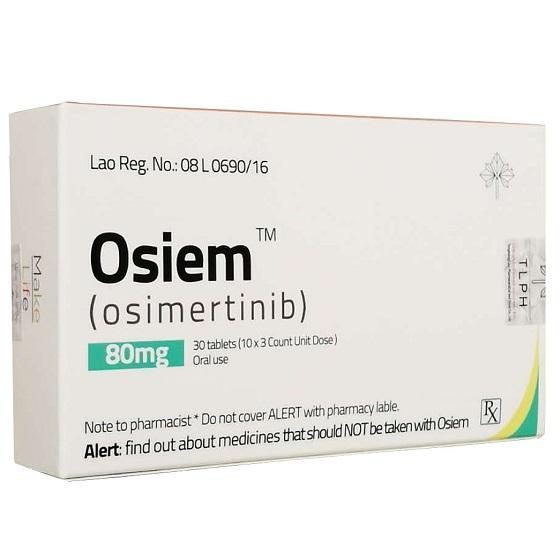
Packaging Sizeo8mg830Tablets/Box
-
Strength80mg
-
CompositonOsimertinib
-
TreatmentEGFR Lung Cancer (Non-small cell lung cancer (NSCLC)
-
FormTablet
-
BrandOsiem
-
Quantity Unit30Tablets/Box
-
ManufacturerTongmeng (Lao) Pharmaceutical & Food Co., Ltd.(TLPH)
[Indications]
It is used for the treatment of advanced patients with metastatic non-small cell lung cancer (NSCLC) with positive EGFRT790M mutation in or after treatment with epidermal growth factor (EGFR) tyrosine kinase inhibitor (TKI).
[How to take the drug]
1. Therapy period
● Before using this product, please confirm the presence of EGFRT790M mutation in the patient's tumor specimens.
● The recommended dose is 80mg/ tablet once a day until the disease progresses or the patient cannot tolerate it.
● Can be taken with or without food.
● If you miss this dose, you don't need to take it again, just take it at the prescribed time next time.
● If the patient has difficulty swallowing, dissolve the product in about 50ml warm boiled water (do not use carbonated drinks) and rinse with clean water at last. Do not crush, heat or ultrasonic crush during dissolution.
2. Dosage form specification
● Tablet, 80mg, 40mg.
3. Contraindications
None.
[Warnings and precautions]
● Interstitial lung disease/pneumonia if the patient develops severe respiratory symptoms during treatment, the product should be suspended and the cause should be investigated promptly. If proved to be interstitial lung disease, the drug is permanently discontinued..
● For those with congenital long QTc syndrome, congestive heart failure, electrolyte disorders, and those who are taking medications that can cause the extension of QTc, regular ecg monitoring and electrolyte chemistry should be performed. In the event of prolonged QTc intervals and the appearance of life-threatening arrhythmia, the drug should be permanently discontinued.
● Cardiomyopathy should be evaluated with an echocardiography or MUGA scan every three months prior to administration and during treatment. If the ratio of LVEF to pre-treatment is reduced by 10% and the value is less than 50%, the drug should be suspended. For symptomatic congestive heart failure or persistent asymptomatic left ventricular dysfunction that does not resolve within four weeks, drug withdrawal is permanent.
● The embryo-fetal toxicity is based on the data of animal experiments and the drug mechanism of this product, which can cause serious harm to the pregnant fetus. Women are advised to use effective contraception while taking the medication and within six weeks of stopping. Men are advised to use effective contraception during treatment and up to four months after withdrawal.
[Side-effect]
Common side effect: diarrhea, nausea, loss of appetite, constipation, rash, dry skin, toxic nails, etc.
[How to store the drug]
Keep this medicine at room temperature (25℃).