- Details
- Description
-
Packaging Size1Bottle/Box
-
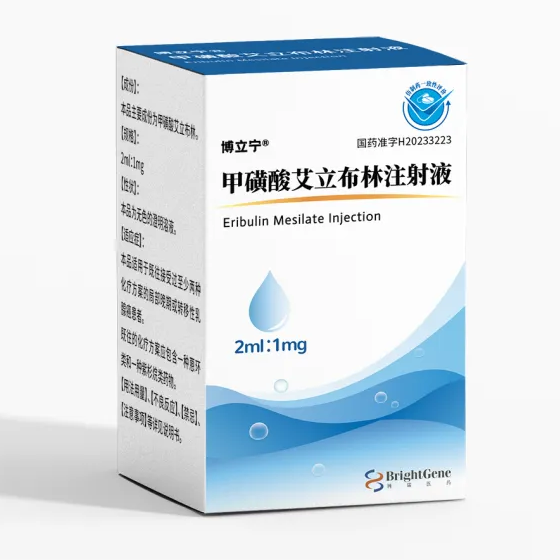
Strength1mg/2mL (0.5mg/mL)
-
CompositonEribulin mesylate
-
TreatmentLiposarcoma,Breast Cancer,
-
FormInjection
-
Brand博立宁®
-
Quantity Unit1mg/2mL*1Bottle/Box
-
ManufacturerBrightGene Bio-Medical Technology Co Ltd
About Eribulin mesylate Injection
Eribulin is a chemotherapy drug. It's a treatment for breast cancer and liposarcoma that has: spread to surrounding tissue (locally advanced cancer) spread to other areas of the body (metastatic or advanced cancer)
- Generic (Eribulin mesylate) sold as brand name 博立宁® in china.
Breast Cancer, Metastatic
Indicated for metastatic breast cancer in patients who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease; prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting
1.4 mg/m² IV infused over 2-5 min on days 1 and 8 of 21-day cycle
Liposarcoma
Indicated for unresectable or metastatic liposarcoma in patients who have received a prior anthracycline-containing regimen
1.4 mg/m² IV infused over 2-5 min on days 1 and 8 of 21-day cycle
Dosage Modifications
Assess for peripheral neuropathy and obtain CBC counts prior to each dose
Recommended dose delays
-
Do not administer on Day 1 or Day 8 for any of the following
- ANC <1,000/mm³
- Platelets <75,000/mm³
- Grade 3 or 4 nonhematological toxicities
-
The Day 8 dose may be delayed for a maximum of 1 week:
- If toxicities do not resolve or improve to ≤Grade 2 severity by Day 15, omit the dose
- If toxicities resolve or improved to ≤Grade 2 severity by Day 15, administer at a reduced dose and initiate the next cycle no sooner than 2 weeks later
Recommended dose reductions
- If a dose has been delayed for toxicity and then recovered to ≤Grade 2 severity, resume at reduced doses (see below)
- Do not re-escalate once dose has been reduced
-
Permanently reduce the 1.4 mg/m² dose to 1.1 mg/m² for any of the following:
- ANC <500/mm³ for >7 days
- ANC <1,000/mm³ with fever or infection
- Platelets <25,000/mm³
- Platelets <50,000/mm³ requiring transfusion
- Nonhematologic Grade 3 or 4 toxicities
- Omission or delay of Day 8 dose in previous cycle for toxicity
-
Permanently reduce dose to 0.7 mg/m² for any of the following:
- Occurrence of any event requiring permanent dose reduction while receiving 1.1 mg/m²
-
Discontinue
- Occurrence of any event requiring permanent dose reduction while receiving 0.7 mg/m²
Renal impairment
- Moderate-to-severe (CrCl 15-49 mL/min): 1.1 mg/m² IV
Hepatic impairment
- Mild (Child-Pugh A): 1.1 mg/m² IV
- Moderate (Child-Pugh B): 0.7 mg/m² IV