- Details
- Description
-
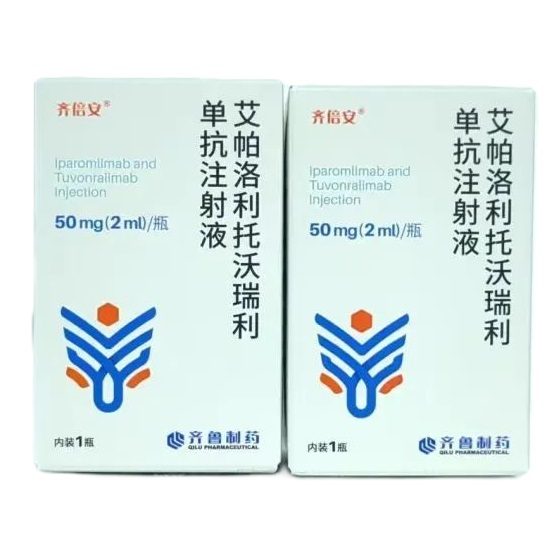
Packaging Size1vail Bottle
-
Strength50mg (2ml)
-
CompositonIparomlimab/tuvonralimab
-
TreatmentRecurrent or metastatic cervical cancer
-
FormInjection
-
Brand齐倍安
-
Quantity Unit50mg (2ml)*1/bottle/Box
-
ManufacturerQilu Pharmacy Limited Company.,China
About Iparomlimab/Tuvonralimab
- Iparomlimab/Tuvonralimab sold under the brand name 齐倍安® in china.
On September 30, 2024, China's National Medical Products Administration (NMPA) announced the conditional approval of (Iparomlimab/tuvonralimab) injection for the treatment of patients with recurrent or metastatic cervical cancer who have failed previous platinum-containing chemotherapy.