- Details
- Description
-
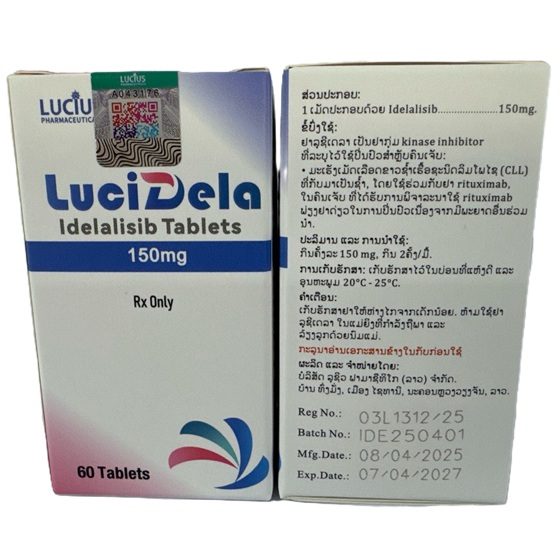
Packaging Size60t
-
Strength150mg
-
CompositonIdelalisib
-
TreatmentChronic Lymphocytic Leukemia(CLL)、Small Lymphocytic Lymphoma(SLL)、Follicular B-cell Non-Hodgkin Lymphoma
-
FormTablet
-
BrandLuciDela
-
Quantity Unit150mg*60t/Box
-
ManufacturerLucius Pharmaceuticals (Lao) Co.,Ltd
About Idelalisib
Idelalisib is a medication used to treat certain blood cancers. Idelalisib acts as a phosphoinositide 3-kinase inhibitor; more specifically, it blocks P110δ, the delta isoform of the enzyme phosphoinositide 3-kinase.
Chronic Lymphocytic Leukemia
Indicated, in combination with rituximab, for relapsed chronic lymphocytic leukemia (CLL) in patients for whom rituximab alone would be considered appropriate therapy due to other comorbidities
Starting dose: 150 mg PO BID; continue treatment until disease progression or unacceptable toxicity
Follicular B-cell Non-Hodgkin Lymphoma
January 14, 2022: Indication for relapsed follicular B-cell non-Hodgkin lymphoma withdrawn
Unable to enroll enough patients into a confirmatory trial required for the accelerated approval
Small Lymphocytic Lymphoma
January 14, 2022: Indication for small lymphocytic lymphoma withdrawn
Unable to enroll enough patients into a confirmatory trial required for the accelerated approval
Dosage Modifications
Pneumonitis: Discontinue with any severity of symptomatic pneumonitis
CrCl ≥15 mL/min: No dose adjustment required
ALT/AST
- >3-5 x ULN: Maintain dose; monitor at least weekly until ≤1 x ULN
- >5-20 x ULN: Withhold idelalisib; monitor ALT/AST at least weekly until ≤1 x ULN, then resume at 100 mg BID
- >20 x ULN: Permanently discontinue
Bilirubin
- >1.5-3 x ULN: Maintain dose; monitor at least weekly until≤1 x ULN
- >3-10 x ULN: Withhold idelalisib; monitor bilirubin at least weekly until ≤1 x ULN, then resume at 100 mg BID
- >10 x ULN: Permanently discontinue
Diarrhea
- Moderate: Maintain dose; monitor at least weekly until resolved
- Severe or hospitalization: Withhold idelalisib; monitor at least weekly until resolved, then resume dose at 100 mg BID
- Life-threatening: Permanently discontinue
Neutropenia
- ANC 1 to <1.5 Gi/L: Maintain dose
- ANC 0.5 to <1 Gi/L: Maintain dose; monitor ANC at least weekly
- ANC <0.5 Gi/L: Withhold idelalisib; monitor ANC at least weekly until ANC ≥0.5 Gi/L, then resume at 100 mg BID
Thrombocytopenia
- Platelets 50 to <75 Gi/L: Maintain dose
- Platelets 25 to <5 Gi/L: Maintain dose; monitor platelets at least weekly
- Platelets <25 Gi/L: Withhold idelalisib; monitor platelets at least weekly until platelets ≥25 Gi/L, then resume at 100 mg BID
Infections
-
Evidence of CMV infection or viremia
- Interrupt idelalisib in patients with evidence of active CMV infection of any grade or viremia (positive PCR or antigen test) until the viremia has resolved
- If idelalisib is resumed, monitor patients by PCR or antigen test for CMV reactivation at least monthly
-
Grade ≥3 sepsis or pneumonia
- Interrupt idelalisib with until infection has resolved
-
Evidence of PJP infection
- Interrupt idelalisib in patients with suspected PJP infection of any grade
- Permanently discontinue idelalisib if PJP infection is confirmed
Other severe or life-threatening toxicities
- Withhold drug until toxicity is resolved
- If resuming treatment after interruption for other severe or life-threatening toxicities, reduce dose to 100 mg twice daily
- Discontinue idelalisib permanently for recurrence of other severe or life-threatening idelalisib-related toxicity upon rechallenge
Dosing Considerations
Monitoring
- Monitor blood counts at least q2 weeks for first 6 months, and at least weekly if neutrophil counts <1x 109/L
- Monitor ALT and AST q2 weeks for first 3 months, q4 weeks for next 3 months, then q1-3 months thereafter