- Details
- Description
-
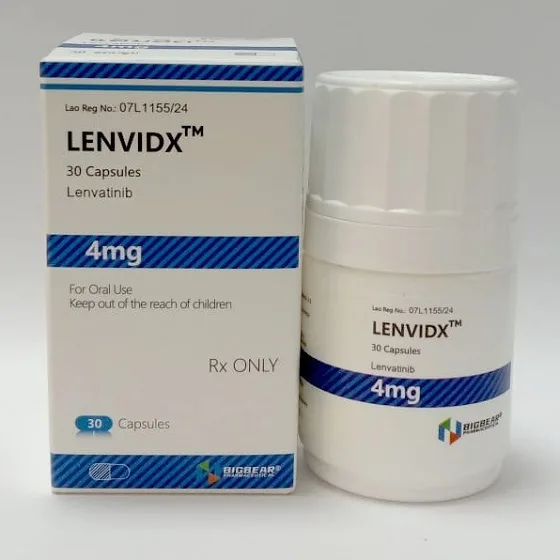
Packaging Size30C/bottle
-
Strength4mg
-
CompositonLenvatinib
-
TreatmentDifferentiated thyroid cancer (DTC),Renal Cell Carcinoma (RCC),Hepatocellular Carcinoma (HCC),Endometrial Cancer,
-
FormCapsule
-
BrandLENVIDX
-
Quantity Unit4mg*30c/bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
About Lenvatinib
Lenvatinib is in a class of medications called kinase inhibitors. It works by blocking the action of an abnormal protein that signals cancer cells to multiply.
Differentiated Thyroid Cancer
Indicated for patients with locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer (DTC)
24 mg (two 10 mg capsules and one 4 mg capsule) PO qDay
Renal Cell Carcinoma
Combination therapy with everolimus
- Indicated in combination with everolimus for the treatment of patients with advanced renal cell carcinoma (RCC) following one prior anti-angiogenic therapy
- Lenvatinib 18 mg (one 10 mg capsule and two 4 mg capsules) PO qDay PLUS
- Everolimus 5 mg PO qDay
- Continue until disease progression or until unacceptable toxicity
Combination therapy with pembrolizumab
- Indicated in combination with pembrolizumab for first-line treatment of patients with advanced RCC
- Lenvatinib 20 mg PO qDay, PLUS
- Pembrolizumab 200 mg IV q3Weeks OR 400 mg q6Weeks
- Continue until disease progression, unacceptable toxicity, or for pembrolizumab, up to 24 months in patients without disease progression
Hepatocellular Carcinoma
Indicated for first-line treatment of patients with unresectable hepatocellular carcinoma (HCC)
Dose based on actual body weight
- <60 kg: 8 mg PO qDay
- ≥60 kg: 12 mg PO qDay
- Continue until disease progression or until unacceptable toxicity
Endometrial Cancer
Indicated in combination with pembrolizumab for patients with advanced endometrial carcinoma that is not microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), who have disease progression following prior systemic therapy and are not candidates for curative surgery or radiation
20 mg PO qDay, PLUS pembrolizumab 200 mg IV q3weeks
Continue until disease progression or unacceptable toxicity
Refer to pembrolizumab prescribing information for recommended dosing information
Dosage Modifications
DTC dose reductions
- First occurrence: Reduce to 20 mg PO qDay
- Second occurrence: Reduce to 14 mg PO qDay
- Third occurrence: Reduce to 10 mg PO qDay
RCC or endometrial carcinoma dose reductions
- First occurrence: Reduce to 14 mg PO qDay
- Second occurrence: Reduce to 10 mg PO qDay
- Third occurrence: Reduce to 8 mg PO qDay
-
Dose modification of everolimus in RCC
- Reduce lenvatinib dose first and then the everolimus dose for adverse reactions of both lenvatinib and everolimus
- Refer to the everolimus prescribing information for additional dose modification information
-
Dose modification of pembrolizumab in endometrial carcinoma
- Interrupt one or both drugs or reduce lenvatinib when appropriate
- No dose reductions are recommended for pembrolizumab
- Refer to pembrolizumab prescribing information for additional dose modification information
HCC dose reductions
-
First occurrence
- <60 kg: Reduce to 4 mg PO qDay
- ≥60 kg: Reduce to 8 mg PO qDay
-
Second occurrence
- <60 kg: Reduce to 4 mg PO every other day
- ≥60 kg: Reduce to 4 mg PO qDay
-
Third occurrence
- <60 kg: Discontinue
- ≥60 kg: Reduce to 4 mg PO every other day
Hypertension
- Grade 3: Withhold if persists despite optimal antihypertensive therapy; resume at reduced dose when hypertension is controlled at Grade ≤2
- Grade 4: Permanently discontinue
Cardiac dysfunction
- Grade 3: Withhold until improved Grade≤1or baseline; resume at reduced dose or discontinue depending on severity and persistence of adverse reaction
- Grade 4: Permanently discontinue
Arterial thrombotic event
- Any grade: Permanently discontinue
Hepatoxicity
- Grade 3 or 4: Withhold until improved Grade ≤1 or baseline; resume at reduced dose or discontinue depending on severity and persistence of adverse reaction
- Hepatic failure: Permanently discontinue
Proteinuria
- ≥2 g/24 hr: Withhold; resume at reduced dose after resolution to <2 g/24 hr
- Nephrotic syndrome: Permanently discontinue
GI toxicity
- Grade 3 nausea, vomiting, and diarrhea: Withhold; resume at reduced dose after resolution to Grade ≤1 or baseline
- Grade 4 nausea, vomiting, and diarrhea: Permanently discontinue
- GI perforation, any grade: Permanently discontinue
- Grade 3 or 4 GI fistula: Permanently discontinue
Renal failure or impairment
- Grade 3 or 4: Withhold until improved Grade ≤1 or baseline; resume at reduced dose or discontinue depending on severity and persistence of adverse reaction
QTc prolongation
- >500 ms or >60 ms increase from baseline: Withhold; resume at reduced dose after resolution to ≤480 ms or baseline
Reversible posterior leukoencephalopathy syndrome (RPLS)
- Any grade: Withhold until fully resolved, resume at reduced dose or discontinue depending on severity and persistence of neurologic symptoms
Other adverse reactions
- Persistent or intolerable Grade 2 or 3 adverse reaction, or Grade 4 laboratory abnormality: Withhold until improves to Grade ≤1 or baseline; resume at reduced dose
- Grade 4 adverse reaction: Permanently discontinue
Renal impairment
- Mild-to-moderate (CrCl ≥30 mL/min): No dosage adjustment necessary
-
Severe (CrCl <30 mL/min)
- DTC: 14 mg PO qDay
- RCC and endometrial carcinoma: 10 mg PO qDay
- Endometrial carcinoma: 10 mg PO qDay
Hepatic impairment
- Mild-to-moderate (Child-Pugh A or B): No dosage adjustment necessary
-
Severe (Child-Pugh C)
- DTC: 14 mg PO qDay
- RCC and endometrial carcinoma: 10 mg PO qDay
- Endometrial carcinoma: 10 mg PO qDay