- Details
- Description
-
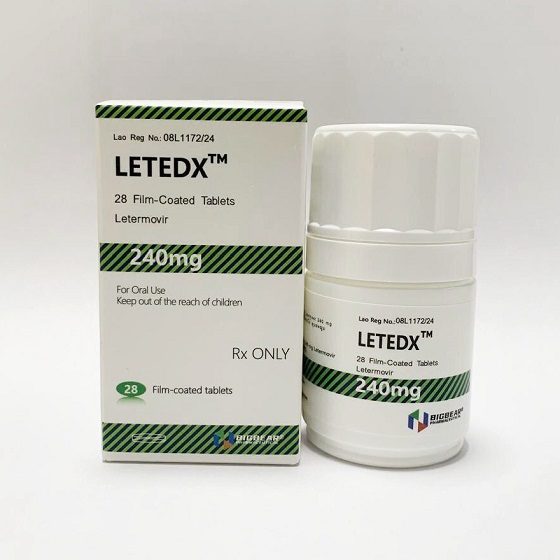
Packaging Size28T/Bottle
-
Strength240mg
-
CompositonLetermovir
-
TreatmentCytomegalovirus (CMV) infections
-
FormTablet
-
BrandLETEDX
-
Quantity Unit240mg*28t/Bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
About Letermovir
Letermovir is an antiviral drug for the treatment of cytomegalovirus (CMV) infections. It has been tested in CMV infected patients with allogeneic stem cell transplants and may also be useful for other patients with a compromised immune system such as those with organ transplants or HIV infections.
Adult
Cytomegalovirus Infection Prophylaxis in HSCT
Indicated for prophylaxis of cytomegalovirus (CMV) infection and disease in adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant (HSCT)
480 mg PO/IV (one 480 mg tab or two 240 mg tabs) qDay; may use four 120 mg packets of oral pellets qDay for patient who cannot swallow tablets; initiate between Day 0 and Day 28 posttransplantation (before or after engraftment) and continue through Day 100
May continue through Day 200 post HSCT for patients at risk for late CMV infection and disease
Cytomegalovirus Disease Prophylaxis in Kidney Transplant Recipients
Indicated for prophylaxis of cytomegalovirus (CMV) disease in adult kidney transplant recipients at high risk (donor CMV seropositive/recipient CMV seronegative [D+/R-])
480 mg PO/IV (one 480 mg tab or two 240 mg tabs) qDay; may use four 120 mg packets of oral pellets qDay; initiate between Day 0 and Day 7 post-transplant and continue through Day 200 post-transplant
Dosage Modifications
Coadministration with cyclosporine
- Decrease letermovir dose to 240 mg/day if coadministered with cyclosporine (monitor cyclosporine levels)
- If cyclosporine initiated after letermovir, decrease the next letermovir dose to 240 mg/day
- If cyclosporine discontinued after starting letermovir, increase the next letermovir dose to 480 mg/day
- If cyclosporine dosing is interrupted because of high cyclosporine levels, no dose adjustment of letermovir is needed
Renal impairment
- CrCl >10 mL/min: No dosage adjustment required
- CrCl ≤10 mL/min or patient on dialysis: Data are insufficient to make dosing recommendations
- Hydroxypropyl betadex (IV vehicle) may accumulate if CrCl <50 mL/min; closely monitor serum creatinine levels in patients receiving IV letermovir
Hepatic impairment
- Mild or moderate (Child-Pugh A or B): No dosage adjustment required
- Severe (Child-Pugh C): Not recommended
Dosing Considerations
Following prophylaxis completion, monitoring for CMV reactivation recommended
Use IV only in patients unable to take oral therapy; switch to oral administration as soon as feasible
Tablet and injection may be used interchangeably at the discretion of the physician; no dosage adjustment is necessary when switching formulations
Pediatric
Cytomegalovirus infection prophylaxis in HSCT
Indicated for prophylaxis of cytomegalovirus (CMV) infection and disease in adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant (HSCT)
>12 years
- >=30 kg: 480 mg (one 480 mg tab or two 240 mg tabs) PO/IV qDay; may use four 120 mg packets of oral pellets qDay for patient who cannot swallow tablets
>=6 years:
- 15-30 kg: 240 mg (one 240 mg tab or two 120 mg packets o) PO qDay; may use four 120 mg packets of oral pellets qDay or 120 mg IV qDay
- 7.5 to < 30 kg: tablets not recommended; 120 mg packets o) PO qDay; may use one 120 mg packets of oral pellets qDay or 60 mg IV qDay
- 6 to <7.5 kg: tablets not recommended; four 20 mg packets of oral pellets PO qDay or 40 mg IV qDay
- Initiate between Day 0 and Day 28 posttransplantation (before or after engraftment) and continue through Day 100
- May continue through Day 200 post HSCT for patients at risk for late CMV infection and disease
Cytomegalovirus disease prophylaxis in kidney transplant recipients
Indicated for prophylaxis of cytomegalovirus (CMV) disease in adult kidney transplant recipients at high risk (donor CMV seropositive/recipient CMV seronegative [D+/R-])
>12 years
- >=30 kg: 480 mg (one 480 mg tab or two 240 mg tabs) PO/IV qDay; may use four 120 mg packets of oral pellets qDay for patient who cannot swallow tablets
>=6 years:
- 15-30 kg: 240 mg (one 240 mg tab or two 120 mg packets o) PO qDay; may use four 120 mg packets of oral pellets qDay or 120 mg IV qDay
- 7.5 to < 30 kg: tablets not recommended; 120 mg packets o) PO qDay; may use one 120 mg packets of oral pellets qDay or 60 mg IV qDay
- 6 to <7.5 kg: tablets not recommended; four 20 mg packets of oral pellets PO qDay or 40 mg IV qDay
- Initiate between Day 0 and Day 7 post-transplant and continue through Day 200 post-transplant
Dosage modifications
Coadministration with cyclosporine in HSCT patients (>=12 years and >=30 kg) and kidney transplant (>=12 years and >=40 kg)
- Decrease letermovir dose to 240 mg/day if coadministered with cyclosporine (monitor cyclosporine levels)
- If cyclosporine initiated after letermovir, decrease the next letermovir dose to 240 mg/day
- If cyclosporine discontinued after starting letermovir, increase the next letermovir dose to 480 mg/day
- If cyclosporine dosing is interrupted because of high cyclosporine levels, no dose adjustment of letermovir is needed
Co-administered with cyclosporine in HSCT recipients 6 Months to <12 years or >=12 Years and <30 kg
- 15 to <30 kg: Tablets not recommended; one 120 mg packet of oral pellets PO qDay or 120 mg IV qDay
- 7.5 to < 15 kg: Tablets not recommended; three 20 mg packets of oral pellets PO qDay ot 60 mg IV qDay
- 6 to 7.5 kg: Tablets not recommended; two 20 mg packets of oral pellets or 40 mg IV qDay
Renal impairment
- CrCl >10 mL/min: No dosage adjustment required
- CrCl ≤10 mL/min or patient on dialysis: Data are insufficient to make dosing recommendations
- Hydroxypropyl betadex (IV vehicle) may accumulate if CrCl <50 mL/min; closely monitor serum creatinine levels in patients receiving IV letermovir
Dosing considerations
- Following prophylaxis completion, monitoring for CMV reactivation recommended
- Use IV only in patients unable to take oral therapy; switch to oral administration as soon as feasible
- Tablet and injection may be used interchangeably at the discretion of the physician; no dosage adjustment is necessary when switching formulations
- Dosage adjustment may be necessary for pediatric patients <12 years of age when switching between oral and intravenous formulations