- Details
- Description
-
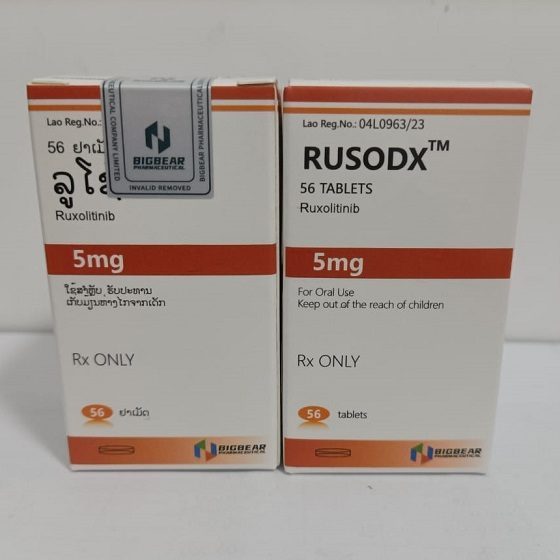
Packaging Size30T/bottle
-
Strength5mg
-
CompositonRuxolitinib
-
TreatmentMyelofibrosis,Polycythemia Vera,Acute Graft versus Host Disease,Chronic Graft versus Host Disease
-
FormTablet
-
BrandRUSODX
-
Quantity Unit5mg*56T/bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
Ruxolitinib is a medication used for the treatment of intermediate or high-risk myelofibrosis,a type of myeloproliferative neoplasm that affects the bone marrow; polycythemia vera, when there has been an inadequate response to or intolerance of hydroxyurea; and steroid-refractory acute graft-versus-host disease. Ruxolitinib is a Janus kinase inhibitor.
Myelofibrosis
Kinase inhibitor indicated for treatment of patients with intermediate or high-risk myelofibrosis (MF), including primary MF, post-polycythemia vera MF and post-essential thrombocythemia MF
Initial dose
- Platelet count >200 x109/L: 20 mg PO BID
- Platelet count 100-200 x109/L: 15 mg PO BID
- Platelet count 50 to <100 x109/L: 5 mg PO BID
Insufficient response for patients starting treatment with a platelet count ≥100 X 10^9/L
- If response is insufficient and platelet count and ANC are adequate, dose may be increased in 5 mg BID increments; not to exceed 25 mg PO BID
- Do not increase dose during the first 4 weeks of therapy and no more frequently than q2Weeks
-
Consider dose increases in patients who meet all of the following conditions:
- Failure to achieve a reduction from pretreatment baseline in either palpable spleen length of 50% or a 35% reduction in spleen volume as measured by CT or MRI
- Platelet count >125 x109/L at 4 weeks and never <100 x109/L
- ANC levels >0.75 x109/L
- Long-term maintenance at 5 mg BID has not shown responses and limit continued use at this dose to patients in whom the benefits outweigh the potential risks
- Discontinue if there is no spleen size reduction or symptom improvement after 6 months of therapy
Insufficient response for patients starting treatment with a platelet count 50 x 10^9/L to <100 x 10^9/L
- If response is insufficient and platelet count and ANC are adequate, doses may be increased by increments of 5 mg qDay to up to 10 mg BID
- Do not increase dose during the first 4 weeks of therapy and no more frequently than q2Weeks
-
Consider dose increases in patients who meet all of the following conditions:
- Platelet count has remained at least 40 x 109/L
- Platelet count has not fallen by >20% in the prior 4 weeks
- ANC >1 x 109/L
- No dose reduction or interruption for an adverse event or hematological toxicity in the prior 4 weeks
- Continuation of treatment for >6 months should be limited to patients in whom the benefits outweigh the potential risks
- Discontinue treatment if there is no spleen size reduction or symptom improvement after 6 months of therapy
Polycythemia Vera
Indicated for polycythemia vera in patients who have had an inadequate response to or are intolerant of hydroxyurea
Initial: 10 mg PO BID
Increasing dose for insufficient response
- If response is insufficient and platelet, hemoglobin, and neutrophil counts are adequate, doses may be increased in 5 mg BID increments to a maximum of 25 mg BID
- Doses should not be increased during the first 4 weeks of therapy and not more frequently than q2Week
-
Consider dose increases in patients who meet all of the following criteria:
- Inadequate efficacy as demonstrated by ≥1 of the following: continued need for phlebotomy, WBC > ULN, platelet count >ULN, palpable spleen that is reduced by <25% from baseline
- Platelet count ≥140 x109/L
- Hemoglobin ≥2 g/dL
- ANC ≥1.5 x 109/L
Acute Graft versus Host Disease
Indicated for treatment of steroid-refractory acute graft-versus-host disease (GVHD)
Initial dose: 5 mg PO BID; may increase to 10 mg BID after at least 3 days if ANC and platelet counts have not decreased by ≥50% compared to baseline
Consider tapering after 6 months in patients with response who have discontinued therapeutic doses of corticosteroids
Tapering dose
- Taper by 1 dose level ~q8Weeks (eg, 10 mg BID to 5 mg BID to 5 mg qDay)
- Patients unable to tolerate 5 mg qDay: Interrupt treatment until clinical and/or laboratory parameters recover
- If acute GVHD signs or symptoms recur during or after taper, consider retreatment
Chronic Graft versus Host Disease
Indicated for treatment of chronic graft-versus-host disease (cGVHD) after failure of 1 or 2 lines of systemic therapy
Initial dose: 10 mg PO BID
Consider tapering after 6 months in patients with response who have discontinued therapeutic doses of corticosteroids
Tapering dose
- Taper by 1 dose level ~q8Weeks (eg, 10 mg BID to 5 mg BID, then from 5 mg BID to 5 mg qDay)
- If acute GVHD signs or symptoms recur during or after taper, consider retreatment