- Details
- Description
-
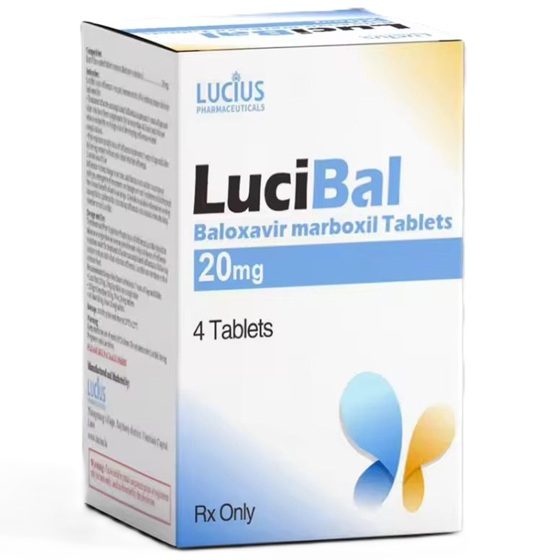
Packaging Size4t/bottle/Box
-
Strength20mg
-
CompositonLuciBal (Baloxavir Marboxil)
-
Treatmentinfluenza A and influenza B
-
FormTablet
-
BrandLuciBal (Baloxavir Marboxil)
-
Quantity Unit20mg*4t/box
-
ManufacturerLucius Pharmaceuticals (Lao) Co.,Ltd
About Baloxavir marboxil
Baloxavir marboxil is an antiviral medication used for the treatment of influenza A and influenza B. It can reduce the duration of flu symptoms by about 1-2 days in some people, but can also develop selection of resistant mutants that render it ineffectual; however studies noted this was seen mostly in children that were studied.
Influenza
Indications
- Indicated for treatment of acute uncomplicated influenza in patients who have been symptomatic for ≤48 hr and who are otherwise healthy or at high risk of developing influenza-related complications
- Indicated for postexposure prophylaxis of influenza following contact with an individual who has influenza
Dosage
-
Tablet or granules for oral suspension
- <80 kg: 40 mg PO as a single dose
- ≥80 kg: 80 mg PO as a single dose
Dosage Modifications
Coadministration with polyvalent cation-containing products
- Avoid coadministration with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives or antacids, or oral supplements (eg, calcium, iron, magnesium, selenium, zinc)
Renal impairment
- CrCl ≥50 mL/min: Pharmacokinetic analysis did not identify a clinically meaningful effect
- Severe: Not studied
Hepatic impairment
- Moderate (Child-Pugh B) to normal: No clinically meaningful pharmacokinetic differences were observed
- Severe: Not studied
Dosing & Uses
Influenza
Indications
- Indicated for treatment of acute uncomplicated influenza in patients who have been symptomatic for ≤48 hr and who are otherwise healthy or at high risk of developing influenza-related complications
- Indicated for postexposure prophylaxis of influenza following contact with an individual who has influenza
Dosage
-
Tablet or granules for oral suspension
- <80 kg: 40 mg PO as a single dose
- ≥80 kg: 80 mg PO as a single dose
Dosage Modifications
Coadministration with polyvalent cation-containing products
- Avoid coadministration with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives or antacids, or oral supplements (eg, calcium, iron, magnesium, selenium, zinc)
Renal impairment
- CrCl ≥50 mL/min: Pharmacokinetic analysis did not identify a clinically meaningful effect
- Severe: Not studied
Hepatic impairment
- Moderate (Child-Pugh B) to normal: No clinically meaningful pharmacokinetic differences were observed
- Severe: Not studied
Dosing Considerations
Limitations of use
- Influenza viruses change over time, and factors (eg, virus type or subtype, emergence of resistance, changes in viral virulence) could diminish drug’s clinical benefit
- Check drug susceptibility patterns for circulating influenza virus strains