- Details
- Description
-
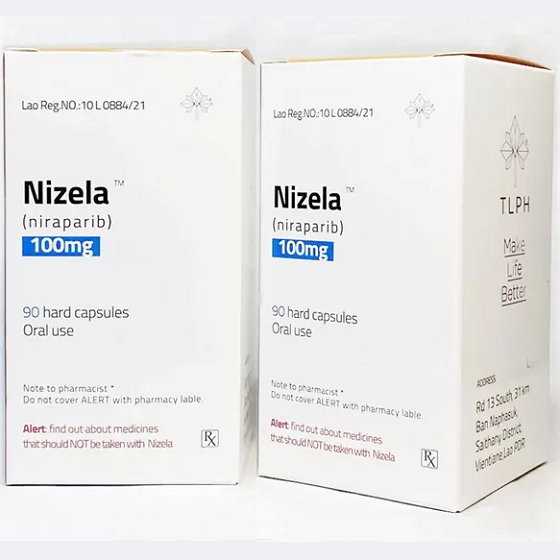
Packaging Size90C/bottle
-
Strength100mg
-
CompositonNiraparib
-
TreatmentOvarian Cancer
-
FormCapsule
-
BrandNizela
-
Quantity Unit100mg*90C/Bottle
-
ManufacturerTongmeng (Lao) Pharmaceutical & Food Co., Ltd.(TLPH)
Niraparib is an anti-cancer medication used for the treatment of epithelial ovarian, fallopian tube, or primary peritoneal cancer.It is taken by mouth. It is a PARP inhibitor.
The most common side effects include nausea (feeling sick), thrombocytopenia (low blood platelet counts), tiredness and weakness, anemia (low red blood cell counts), constipation, vomiting, abdominal (belly) pain, neutropenia (low levels of neutrophils, a type of white blood cell), insomnia (difficulty sleeping), headache, lack of appetite, diarrhea, dyspnea (difficulty breathing), hypertension (high blood pressure), back pain, dizziness, cough, joint pain, hot flushes and decrease in white blood cells.
Niraparib was approved for medical use in the United States and in the European Union in 2017.
Medical uses
Niraparib is indicated for the maintenance treatment of adults with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to platinum-based chemotherapy.
In October 2019, the indication for niraparib was expanded to include people with advanced ovarian, fallopian tube, or primary peritoneal cancer treated with three or more prior chemotherapy regimens and whose cancer is associated with homologous recombination deficiency (HRD)-positive status. HRD is defined by either a deleterious or suspected deleterious BRCA mutation, or genomic instability in patients with disease progression greater than six months after response to the last platinum-based chemotherapy.
In April 2020, the indication for niraparib was expanded to include the maintenance treatment of adults with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to first-line platinum-based chemotherapy.
In the European Union, niraparib is indicated: as monotherapy for the maintenance treatment of adults with advanced epithelial (FIGO Stages III and IV) high-grade ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy; and as monotherapy for the maintenance treatment of adults with platinum sensitive relapsed high grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum based chemotherapy.
Why is this medication prescribed?
Niraparib is used to help maintain the response of certain types of ovarian (female reproductive organs where eggs are formed), fallopian tube (tube that transports eggs released by the ovaries to the uterus), and peritoneal (layer of tissue that lines the stomach) cancer in people who have completely responded or partially responded to other chemotherapy medication(s). It is also used to treat certain types of ovarian, fallopian tube, or peritoneal cancer when the cancer has gotten worse after 3 or more chemotherapy treatments. Niraparib is in a class of medications called poly (ADP-ribose) polymerase (PARP) inhibitors. It works by killing cancer cells.
How should this medicine be used?
Niraparib comes as a capsule to take by mouth once daily. Take niraparib at around the same time every day with or without food. If you experience nausea after taking niraparib, take it once daily at bedtime. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take niraparib exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Swallow the capsules whole; do not open, chew, or dissolve them.
If you vomit after taking niraparib, do not take another dose. Continue your regular dosing schedule.
Your doctor may decrease your dose of niraparib, or permanently or temporarily stop your treatment, if you experience certain side effects. Be sure to tell your doctor how you are feeling during your treatment with niraparib.
Ask your pharmacist or doctor for a copy of the manufacturer's information for the patient.
Other uses for this medicine
This medication may be prescribed for other uses; ask your doctor or pharmacist for more information.