- Details
- Description
-
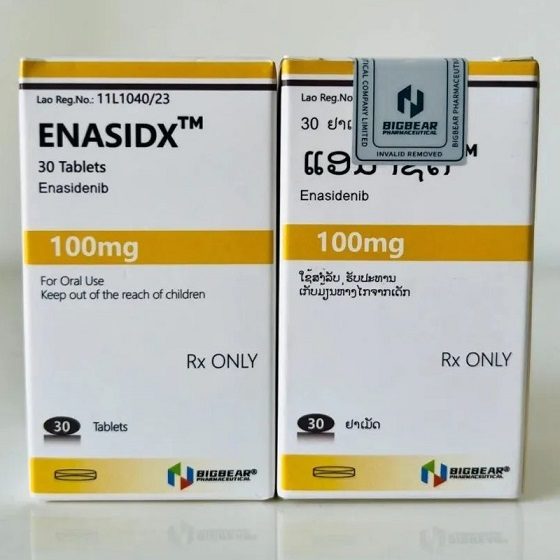
Packaging Size30T/bottle
-
Strength100mg
-
CompositonEnasidenib
-
TreatmentAcute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation
-
FormTablet
-
BrandENASIDX
-
Quantity Unit100mg*30T/bottle
-
ManufacturerBIGBEAR BIGBEAR Pharma,LAOs PDR
Enasidenib is a medication used to treat relapsed or refractory acute myeloid leukemia (AML) in people with specific mutations of the isocitrate dehydrogenase 2 (IDH2) gene, determined by an FDA-approved IDH2 companion diagnostic test. It is an inhibitor of IDH2.
Acute Myeloid Leukemia
Indicated for relapsed/refractory acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation
100 mg PO qDay until disease progression or unacceptable toxicity
In patients without disease progression or unacceptable toxicity, treat for ≥6 months to allow time for clinical response
Dosage Modifications
Differentiation syndrome
- If differentiation syndrome suspected, administer systemic corticosteroids; initiate hemodynamic monitoring
- Interrupt therapy if pulmonary symptoms (eg, intubation/ventilator support) and/or renal dysfunction persists for >48 hr post corticosteroid initiation
- Resume once signs/symptoms improve to Grade ≤2 (mild-moderate)
Noninfectious leukocytosis (WBC >30,000 mcL)
- Initiate hydroxyurea, as per standard institutional guidelines
- Interrupt therapy if no improvement of leukocytosis after initiating hydroxyurea
- Resume at 100 mg/day when WBC <30,000 mcL
Bilirubin elevated >3x ULN
- If sustained for ≥2 weeks without elevated AST/ALT or other hepatic disorders, reduce dose to 50 mg/day
- Resume at 100 mg/day if bilirubin elevation resolves to ≤2x ULN
Other Grade ≥3 toxicities (eg, tumor lysis syndrome)
- Interrupt therapy until toxicity resolve Grade ≤2
- If toxicities resolve (Grade ≤1), resume at 50 mg/day; may increase to 100 mg/day
- If Grade≥3 recurs, discontinue
Dosing Considerations
Monitoring parameters
- Assess baseline blood cell counts and chemistries for leukocytosis and tumor lysis syndrome before initiation; monitor at least q2week for at least first 3 months of treatment
Patient selection
- Select patients for treatment based on presence of IDH2 mutations in the blood and bone marrow