- Details
- Description
-
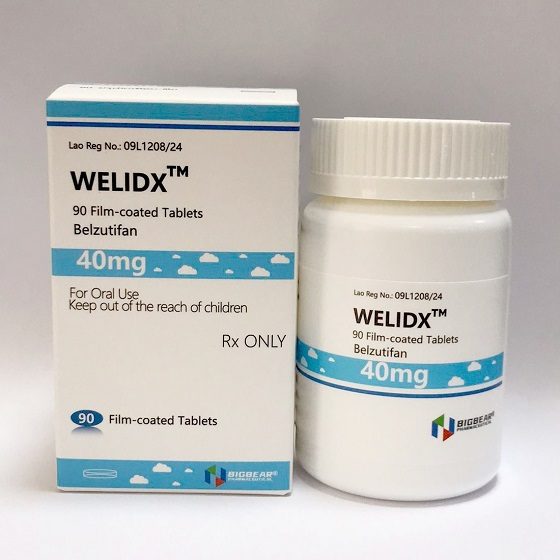
Packaging Size90tablets
-
Strength40mg
-
CompositonBelzutifan
-
TreatmentVon Hippel-Lindau Disease(VHL),Renal Cell Carcinoma(RCC)
-
Formtablet
-
BrandWELIDX
-
Quantity Unit40mg*90t/bottle
-
ManufacturerBIGBEAR Pharma,Laos PDR
About Belzutifan
Belzutifan is an hypoxia-inducible factor-2 alpha inhibitor.
Belzutifan is used to treat von Hippel-Lindau (VHL) disease in patients who need treatment for kidney cancer (renal cell carcinoma), brain and spinal cord tumors (CNS hemangioblastomas), or pancreatic cancer (pancreatic neuroendocrine tumor [pNET]) that do not require surgery right away.
Von Hippel-Lindau Disease
Indicated for adults with von Hippel-Lindau (VHL) disease who require therapy for associated renal cell carcinoma, CNS hemangioblastomas, or pancreatic neuroendocrine tumors, not requiring immediate surgery
120 mg PO qDay
Continue until disease progression or unacceptable toxicity
Renal Cell Carcinoma
Indicated for advanced renal cell carcinoma (RCC) following a programmed death receptor-1 (PD-1) or PD-ligand 1 (PD-L1) inhibitor and a vascular endothelial growth factor tyrosine kinase inhibitor (VEGF-TKI)
120 mg PO qDay
Continue until disease progression or unacceptable toxicity
Dosage Modifications
Dose reductions for adverse reactions
- First dose reduction: 80 mg PO qDay
- Second dose reduction: 40 mg PO qDay
- Third dose reduction: Permanently discontinue
Anemia
- Hemoglobin <9 g/dL or transfusion indicated: Withhold until hemoglobin ≥8 g/dL; resume at same or reduced dose; or discontinue depending on severity of anemia
- Life-threatening or urgent intervention indicated: Withhold until hemoglobin ≥8 g/dL; resume at a reduced dose or permanently discontinue
Hypoxia
- Decreased oxygen saturation with exercise (eg, pulse oximeter <88%): Consider withholding until resolved; resume at same dose or at reduced dose depending on severity
- Decreased oxygen saturation at rest (eg, pulse oximeter <88% or PaO2 ≤55 mm Hg) or urgent intervention indicated: Withhold until resolved; resume at reduced dose or discontinue depending on severity
- Life-threatening or recurrent symptomatic hypoxia: Permanently discontinue
Other adverse reactions
Grade 3: Withhold until resolved to Grade ≤2; consider resuming at a reduced dose (reduce by 40 mg)
Recurrent Grade 3 or Grade 4: Permanently discontinue
Renal impairment
- Mild-to-moderate (eGFR 30-89 mL/min/1.73 m2): No dosage adjustment necessary
- Severe (eGFR 15-29 mL/min/1.73 m2): Not studied
Hepatic impairment
- Mild (total bilirubin [TB] ≤ULN and AST >ULN or TB >1 to 1.5x ULN and any AST): No dosage adjustment necessary
- Moderate-to-severe hepatic impairment (TB >1.5x ULN and any AST): Not studied
Dosing Considerations
Verify pregnancy status in females of childbearing potential before initiation
Closely monitor for adverse reactions in patients who are dual UGT2B17 and CYP2C19 poor metabolizers