FREIENBACH, Switzerland–04.11.2021
EffRx Pharmaceuticals SA, a commercial-stage company that commercializes niche and orphan medicines in Switzerland and Europe, today announced that Swissmedic has approved Bronchitol® (inhaled mannitol) for the treatment of cystic fibrosis (CF) in adults and in children aged 6 years and above as add‐on to other medicines.
The clinical program supporting the registration of Bronchitol® consisted of three large-scale global clinical trials and enrolled a total of 1,065 subjects. Bronchitol® use led to a sustained improvement in FEV1 (Forced Expiratory Volume) versus control. The statistically significant improvement in FEV1 was observed over the 26-week treatment period in those patients receiving Bronchitol® when compared to patients in the control group. The most common (≥1/100, <1/10) adverse reactions include cough, hemoptysis, oropharyngeal pain, vomiting, wheezing and headache.
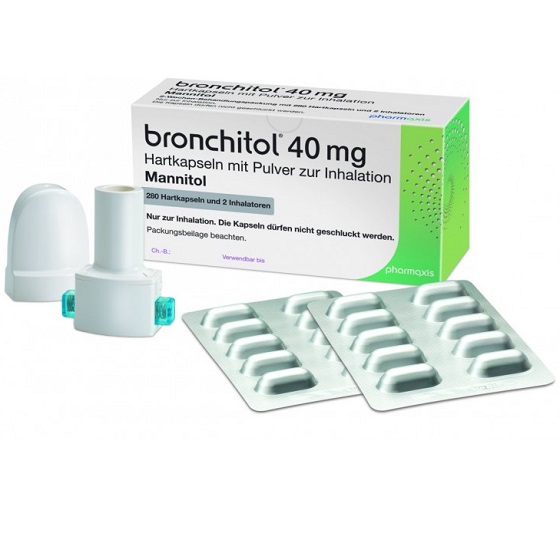
Bronchitol® is a precision spray‐dried form of mannitol that is delivered to the lungs by a specially designed, portable inhaler. Bronchitol® improves lung function and helps to clear mucus from the lungs in patients suffering from cystic fibrosis. Patients are required to pass a tolerance test prior to being prescribed Bronchitol® to ensure that they are not hyperresponsive to mannitol.
Bronchitol® is currently marketed in Europe, Russia, Australia and the United States and was developed by Pharmaxis Ltd, a listed pharmaceutical research company in Australia.
EffRx expects the availability of Bronchitol® in Switzerland in the second half of 2022.
Cystic fibrosis (CF) is an inherited, life-limiting disease that affects the body’s exocrine glands, which produce mucus, saliva, sweat and tears. In the lungs of a CF patient, the thick mucus and the thinning of the airway surface liquid make it nearly impossible for the cilia to clear bacteria from the airway. According to the Swiss Society for Cystic Fibrosis (CFCH), around 320,000 Swiss people are carriers of an altered gene that can cause CF (approximately one in 25 people).
SOURCE
https://www.effrx.com/press-releases/