Basel, 22 October 2021
Roche (SIX: RO, ROG; OTCQX: RHHBY) announced that the U.S. Food and Drug Administration (FDA) has approved Susvimo™ (ranibizumab injection) 100 mg/mL for intravitreal use via ocular implant for the treatment of people with neovascular or “wet” age-related macular degeneration (nAMD) who have previously responded to at least two anti-vascular endothelial growth factor (VEGF) injections.
Neovascular AMD is a potentially blinding condition that requires treatment with eye injections as often as once a month. Susvimo, previously called Port Delivery System with ranibizumab, is the first and only FDA-approved treatment for nAMD that offers as few as two treatments per year.
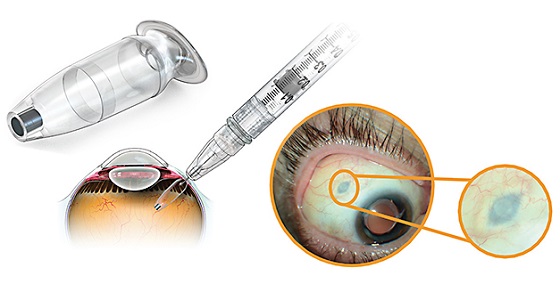
Susvimo delivers ranibizumab continuously, offering people living with nAMD an alternative to anti-VEGF eye injections needed as often as once a month. The implant is surgically inserted into the eye during a one-time, outpatient procedure and refilled every six months.If necessary, supplemental ranibizumab treatment can be given to the affected eye while the Susvimo implant is in place.
The approval is based on positive results from the phase III Archway study primary analysis, which showed nAMD patients treated with Susvimo achieved and maintained vision gains equivalent to monthly ranibizumab injections – +0.2 and +0.5 eye chart letters from baseline, respectively – at weeks 36 and 40 of treatment. In addition, only 1.6% of Susvimo patients received supplemental ranibizumab treatment before their first refill, and more than 98% could go six months before their first refill.
In the Archway study, Susvimo was generally well-tolerated, with a favourable benefit-risk profile. However, the Susvimo implant has been associated with a three-fold higher rate of endophthalmitis than monthly intravitreal injections of ranibizumab. Many of these events were associated with conjunctival retractions or erosions. Appropriate conjunctiva management and early detection with surgical repair of conjunctival retractions or erosions may reduce the risk of endophthalmitis. In clinical trials, 2.0% of patients receiving a ranibizumab implant experienced at least one episode of endophthalmitis. The most common adverse events (AEs) were conjunctival haemorrhage, conjunctival hyperaemia, iritis and eye pain. The safety profile of Susvimo in the clinical trial setting is well understood and will continue to be monitored closely.
Roche has a robust phase III clinical development programme for Susvimo, including the Portal, Pagoda, Pavilion and Velodrome studies. Portal is an extension study evaluating the long-term safety and efficacy of Susvimo in nAMD. Pagoda is evaluating Susvimo for the treatment of people with diabetic macular edema (DME), while Pavilion is a study of Susvimo in diabetic retinopathy without DME. Velodrome is evaluating Susvimo refilled every nine months in nAMD.Susvimo is also currently under review for the treatment of nAMD by the European Medicines Agency (EMA).
Susvimo will be available in the United States in the coming months.
SOURCE
https://www.globenewswire.com/news-release/2021/10/22/2319366/0/en/FDA-approves-Roche-s-Susvimo-a-first-of-its-kind-therapeutic-approach-for-neovascular-or-wet-age-related-macular-degeneration-nAMD.html