BEDFORD, Mass. USA October 11, 2021 Eastern Daylight Time
Ocular Therapeutix, Inc. (NASDAQ:OCUL) , a biopharmaceutical company focused on the formulation, development, and commercialization of innovative therapies for diseases and conditions of the eye, today announced the U.S. Food and Drug Administration (FDA) has approved its Supplemental New Drug Application (sNDA) to broaden the DEXTENZA label to add an additional indication for the treatment of ocular itching associated with allergic conjunctivitis.
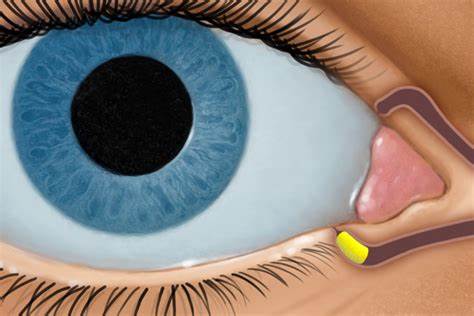
With the approval, DEXTENZA is the first, FDA-approved, physician-administered intracanalicular insert capable of delivering a preservative-free drug for the treatment of ocular itching associated with allergic conjunctivitis with a single administration for up to 30 days.
The efficacy of DEXTENZA for the treatment of ocular itching associated with allergic conjunctivitis was based on three randomized, multicenter, double-masked, parallel group, vehicle-controlled studies in subjects with a positive history of ocular allergies and positive skin test reaction to perennial and seasonal allergens (n=255). In all three trials, DEXTENZA demonstrated lower mean ocular itching scores compared with the vehicle group at all time points throughout the study duration of up to 30 days. In two of the three studies, a higher proportion of patients had statistically significant reductions in ocular itching on Day 8, at 3 minutes, 5 minutes and 7 minutes post-challenge in the DEXTENZA group compared to the vehicle group.
DEXTENZA was observed to have a favorable safety profile and be generally well-tolerated in the allergic conjunctivitis as well as the ocular inflammation and pain clinical populations. The most common ocular adverse events seen in the pooled analysis of the allergic conjunctivitis studies were: increased intraocular pressure (3%), increased lacrimation (1%), eye discharge (1%) and reduced visual acuity (1%). The most common non-ocular adverse reaction that occurred in patients treated with DEXTENZA for allergic conjunctivitis was headache (1%).
About DEXTENZA
DEXTENZA is a corticosteroid intracanalicular insert placed in the punctum, a natural opening in the inner portion of the lower eyelid, and into the canaliculus and is designed to deliver dexamethasone to the ocular surface for up to 30 days without preservatives. DEXTENZA resorbs and exits the nasolacrimal system without the need for removal.
DEXTENZA originally received FDA approval in November 2018 for the treatment of ocular pain following ophthalmic surgery, followed by an expansion of the label to also include the treatment of ocular inflammation following ophthalmic surgery in June 2019.

SOURCE
https://www.businesswire.com/news/home/20211011005575/en