MIAMI, Oct. 19, 2021
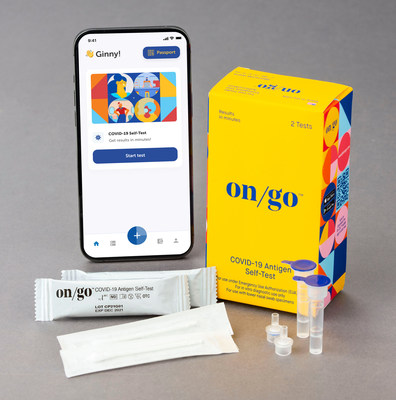
Intrivo announced today the launch of On/Go™, an at-home rapid COVID-19 antigen self-test that delivers results with 95% accuracy in just 10 minutes.
On/Go, which has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA), is an affordable and easy-to-use self-test with a companion app that guides users step-by-step through testing that is highly accurate, fast and convenient.
In addition to direct-to-consumer and enterprise sales, Intrivo was also awarded a U.S. Department of Defense contract to distribute tens of millions of On/Go tests to 25,000 locations across the U.S. that are among the most vulnerable populations in need of rapid testing solutions. These locations include nursing homes, homeless shelters, National Guard sites, military bases and community testing sites.
For population managers, On/Go offers an AI-powered platform that uses Intrivo's proprietary technology to provide fast, accurate and actionable results in the first end-to-end testing-to-tracing COVID-control solution.
The On/Go companion app is available now for free on iOS and Android, and allows for easy viewing, tracking, and sharing of test results. In response to high demand and current shortages of antigen tests, On/Go is readily available for both consumers and enterprises, and Intrivo is ramping up its U.S.-based production rapidly with tests available for order now.
Made in the U.S., On/Go is available for delivery at LetsOnGo.com, Amazon, and Walmart.com.
SOURCE
https://www.prnewswire.com/news-releases/intrivo-launches-ongo-an-fda-authorized-at-home-rapid-covid-19-self-test-delivering-95-accuracy-in-just-10-minutes-through-unique-ai-powered-mobile-app-301402909.html