September 16, 2023 (Vientiane) Laos BIGBEAR Pharmaceuticals announced has recently made a significant stride in the field of immune inflammatory disease treatment with the approval of four breakthrough Janus kinase (JAK) inhibitors. The Lao Health Authority has given the green light to TOFADX, RUSODX, UPADX, and DUVACIDX, marking a new era of treatment options for patients across Asia.
JAK inhibitors are a class of drugs that function by blocking the action of Janus kinases, a group of enzymes that play a crucial role in the immune response. By inhibiting these enzymes, these drugs can help to mitigate the symptoms of immune inflammatory diseases.
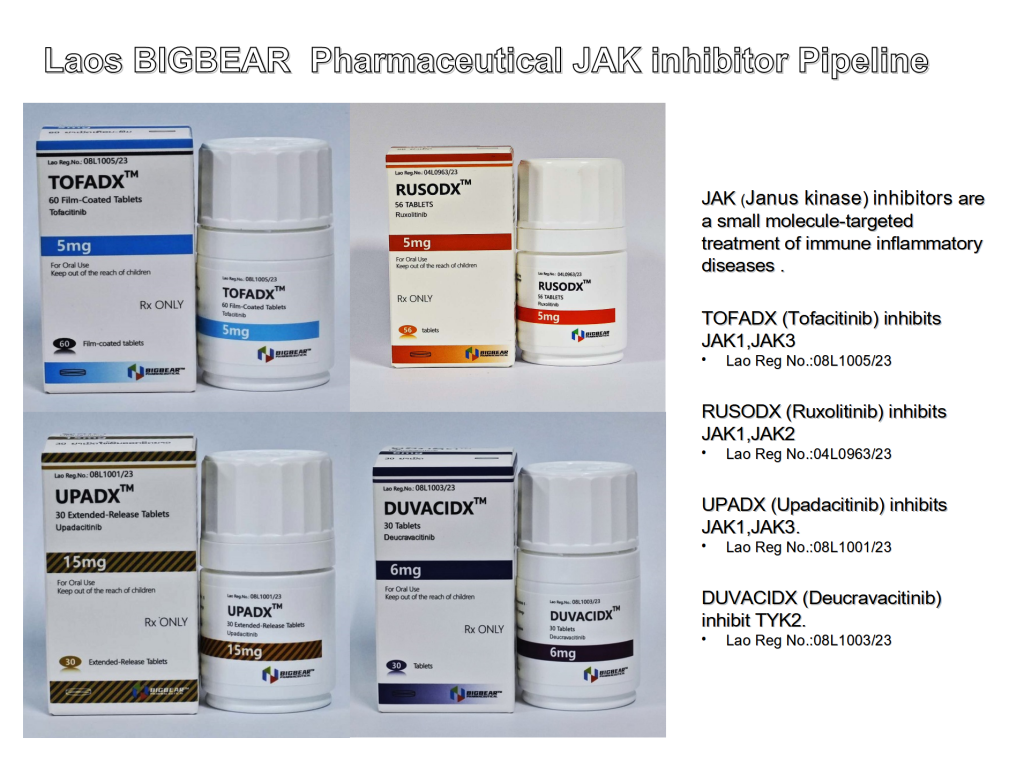
The four approved drugs each target different JAK enzymes, broadening the scope of treatable conditions.
Tofacitinib which inhibits JAK1 and JAK3 enzymes, has been assigned the registration number 08L1005/23. Tofacitinib is typically used in the treatment of moderate to severe rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.
Ruxolitinib inhibiting JAK1 and JAK2, carries the registration number 04L0963/23. Ruxolitinib is primarily used to treat myelofibrosis, a rare type of bone marrow cancer, and polycythemia vera, a slow-growing blood cancer.
Upadacitinib with the registration number 08L1001/23, inhibits JAK1 and JAK3. It is often used for the treatment of moderate to severe rheumatoid arthritis.
Deucravacitinib is a unique JAK inhibitor that targets TYK2. With the registration number 08L1003/23, it is primarily used for the treatment of psoriasis.
The approval of these four JAK inhibitors by the Lao Health Authority signifies an important advancement for immune inflammatory disease treatment across Asia. These drugs offer new hope to patients who have not responded well to traditional treatments.
Moreover, the broad spectrum of conditions these JAK inhibitors can treat, from rheumatoid arthritis to rare bone marrow and blood cancers, means a significant potential improvement in the quality of life for many patients in Asia.
In conclusion, Laos BIGBEAR Pharmaceuticals' commitment to research and development of JAK inhibitors has opened up a new chapter in the treatment of immune inflammatory diseases. The approval of these four drugs not only benefits patients in Laos but also has far-reaching positive implications for countless patients throughout Asia.
Keep your eyes on BIGBEAR Pharma as they continue to innovate and bring new, life-changing treatments to market.