During the global coronavirus(Covid-19) pandemic, we should provide nursing knowledge to ordinary people in underdeveloped countries and regions without access to medical resources in order to improve the survival rate.
This guide is a temporary guide. Please do not refer to it if you can get assistance from medical organizations.
1.Translate
This guide needs to be translated into various languages. This document has been machine translated.
2.Proofreading
It is hoped that more professionals will point out the irrationalities in this guide and add more executable schemes.
3.Contact us
This article has been machine-translated and has not been verified. If there are any errors, please contact us.
Email:care@lienteh.net
For areas where there are no technical conditions, it depends mainly on the following characteristics to determine whether COVID-19 is infected:
All age groups should consider whether COVID-19 is infected if they have a fever and / or cough, accompanied by dyspnea or shortness of breath, chest pain or chest tightness, or unable to speak or act.
1.The most common symptoms of covid-19 are fever and / or respiratory symptoms, including:
2.Other less common symptoms that may affect some patients include:
3.Symptoms of severe covid-19 disease include:
4.Other less common symptoms are:
5.More severe and rare neurological complications, such as:
1.Shortness of breath (increased breathing rate, difficulty breathing, start assisted breathing)
Respiratory rate (RR): the normal respiratory rate of adults is 16 ~ 20 times / min, and that of children is 30 ~ 40 times / min. the respiratory rate of children decreases to the adult level with age. RR was measured by card meter counting.
Adults (over 14 years old): shortness of breath, RR ≥ 30 times / min
Children (under the age of 14): shortness of breath
2.Continuous high fever for more than 3 days
Covid-19 patients receiving care at home should be isolated in a separate room. If this cannot be done, keep a distance of at least 1m from the patients (if there is no separate room, a temporary isolation room can be set up with plastic cloth).
Visitors should not be allowed to enter the home. Patients and anyone else in the same room should wear medical masks.
The patient's isolation room and shared space shall be well ventilated, and the windows shall be opened when it is safe.
Try to select rooms that meet the following conditions as isolation rooms:
Although it is difficult to set up a negative pressure room at home, an isolation room with directional exhaust can be set up.
Inlet air buffer isolation:
A small air buffer chamber shall be set at the entrance of the isolation room with plastic cloth or other high-density materials, and the partition cloth "Π" shall be fully sealed from the roof to the ground. When nurses enter and exit, first open the first layer of door curtain, put down the first layer of door curtain, and then open the second layer of door curtain to slow down the air flow when entering and leaving. The buffer area also serves as a protective equipment unloading area for nursing staff, placing a clothes hanger, disinfection storage cabinet and wash basin.
Door entry pad:
A pad that can absorb liquid is paved on the ground of the air buffer room, which can be made of cotton / hemp and other materials. You can spray "chlorine containing disinfectant" (Reference: environmental disinfection) on the pad to help disinfect the sole.
Buffer room shall be added at the door of toilet:
If you live in a building with new crown patients, set a buffer room at the door of the toilet, and wear a mask when entering the toilet. In addition, inform the residents of the whole building to do so.
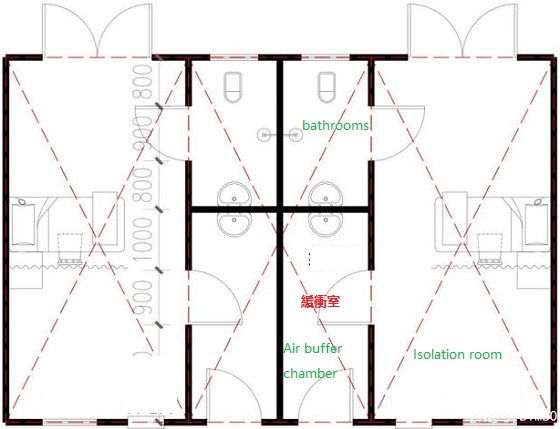
Temporary buffer chamber made of high density fabric
Directional ventilation of electric fan:
Find a household desktop electric fan as the exhaust fan, remove the window glass diagonally / below the door, connect the fan to the window, face the window (windy side), seal the exhaust window with transparent glue or similar materials, turn on the fan power and adjust to the slowest gear.
Narrow tube directional exhaust:
If there is no electric fan, the directional exhaust device can be made by using the narrow tube effect, as shown in the following figure: nped made of waste coke bottles and cardboard. The small pipe opening is the air outlet, and the small pipe opening is facing out of the window. If possible, two npeds can be made, with air inlet from the upwind window and air outlet from the downwind window, so as to speed up the air flow in the isolated room. In tropical areas, nped can also reduce the temperature in the room.

Narrow pipe exhaust device,NPED
COVID-19 patients receiving care at home should be kept isolated. People with symptoms should be isolated for at least 10 days after the first day of symptoms until the disease is cured, and then isolated for another 3 days after the end of symptoms. The end of isolation criteria:
If medical technology equipment is available:
1.Nursing staff:
Set up an independent nursing staff, who can only contact patients. If possible, limit the number of nursing staff to people without basic diseases.
2.Patient's independent daily necessities:
The patient prepares a set of dishes, chopsticks, cups, pots and bedding separately. The patient's food can only be used by the patient and cleaned separately. If there is no chlorine containing disinfectant, use a separate pot as a disinfection pot and boil these daily necessities with boiling water.
3.Boiling disinfection method:
The daily necessities of patients should be boiled with 100 ℃ boiling water for 10 minutes (min). This method is used for the disinfection of general surgical instruments, rubber tubes and syringes, drinking water and tableware.
1.Protective equipment
Nursing staff shall wear the following protective equipment when entering the isolation area:
When the alternative scheme is adopted, wrap all gloves, raincoats, shoe bags, etc. with transparent tape without leaving gaps.
2.Wearing sequence
When nurses and patients are in the same room, they shall not touch their faces during the whole process. After leaving the room, they shall discard masks and wash their hands.
3.Unloading sequence
The buffer zone is used as the unloading area. Two special plastic buckets with covers are prepared in the unloading area. For the unloaded protective equipment, one bucket for disposable equipment and another bucket for reusable equipment.
Disposable medical waste needs high-temperature steam sterilization technology. It is usually placed in a special stainless steel box, pushed into a high-temperature sterilization cabinet, and sterilized for 45 minutes under the pressure of 220 kPa and high temperature and high pressure of 134 ℃. Helmet, goggles and other equipment shall be soaked and cleaned with chlorine containing disinfectant (1000mg / L) and dried before use.
Alternative: the used disposable plastic bags shall be burned in a special brazier. The reusable protective equipment shall be boiled in a special pot for 45 minutes. For equipment such as helmets that cannot be boiled, spray disinfectant alcohol and place them in the sun for 45 minutes.
1.Ultraviolet lamp (if any)
Environmental disinfection: the effective distance shall not exceed 2M and the time shall be 30-60min. Start timing from 5-7min after the lamp is on, because the lamp needs to be preheated, and it takes a certain time to ionize the oxygen in the air to produce ozone.
Disinfection of articles: the irradiation time is 20-30min at an effective distance of 25-60cm.
2.Chlorine containing disinfectant
Chlorinated disinfectant, including:
Scope of use:
1.Thermometer, sphygmomanometer, oxygen tank and other equipment (if any)
2.Daily necessities for patients
1) Patients' wastes, such as paper towels, should be put into closed bags before disposal (special garbage cans and disposable plastic bags, refer to disinfection).
2) Special tableware for patients: plates, cups, tableware, etc. shall be disinfected in the following ways:
3) Bedding and toiletries
Towel, bed sheet, bedding and other fabrics shall be immersed in disinfectant with effective chlorine concentration of 500mg / L for 30 minutes. After disinfection, rinse the residual disinfectant with clean water.
4) Patient excreta
The special barrel shall be used for carrying, and shall not be directly discharged into the sewer.
3.In the isolated room
Surfaces frequently contacted by patients should be cleaned and disinfected at least daily.
4.If there is not enough chlorine containing disinfectant
Alternatives:
If there is no local public health system to deal with such wastes, prepare a box or dig a pit (keep away from the water source), prepare a disinfection isolation layer inside, put the treated wastes into the box, close the box, or bury them in the pit.
The structure is as follows:
If there are the bodies of the unfortunate victims in the local area, they need to be treated on site, burned or buried in the above way.
In the absence of special medical equipment at home, the nursing principles are as follows:
1. bed rest: the patient is resting in the isolation room
2. ensure nutrition and moisture
3.Temperature detection: the normal temperature range is 36°2 ~ 37°3
The drug list provided below, including but not limited to the approval and clinical drug list of local medical regulatory authorities in the United States, the European Union, China, Japan and India, may not be approved locally or the drug cannot be obtained. Therefore, the following list is for reference only.
It is not recommended to use more than three antiviral drugs at the same time. In case of intolerable toxic and side effects, the use of relevant drugs should be stopped. For the treatment of pregnant and parturient patients, we should consider the number of gestational weeks, choose drugs that have little impact on the fetus as far as possible, and consider whether to treat after termination of pregnancy, and inform them.
The following [1-6] are antiviral drugs that have been on the market for many years with relatively high price and accessibility:
1、 Interferon-alpha,IFN-α
For adults, 5 million U or equivalent dose each time, add 2ml of sterile water for injection twice a day, atomize inhalation, and the course of treatment shall not exceed 10 days;
2、Ribavirin+IFN-α(The dose is the same as above)
Ribavirin adult 500mg / time, intravenous infusion 2 to 3 times a day, the course of treatment shall not exceed 10 days;
3、Ribavirin+Lopinavir/Ritonavir
Lopinavir/ritonavir adult 200mg/50mg/capsule, 2 capsules each time, twice a day.
Combined application: ribavirin 500mg / time for adults, intravenous infusion 2 to 3 times a day, and the course of treatment shall not exceed 10 days;
4、Chloroquine Phosphate
For adults aged 18 ~ 65. For those weighing more than 50kg, 500mg each time, twice a day for 7 days; If the body weight is less than 50kg, 500mg each time on the first and second days, twice a day, 500mg each time on the third to seventh days, once a day;
5、Arbidol
Adult 200mg, 3 times a day, the course of treatment shall not exceed 10 days.
6、Azithromycin
On March 19, 2020, the team of Didier raoulta, a French scientist, was accepted by the peer-reviewed scientific journal International Journal of antimicrobial agents. A clinical research paper published showed that hydroxychloroquine sulfate had a significant effect on covid-2019 patients.
In a clinical trial involving 36 patients with covid-2019 (6 asymptomatic, 22 symptomatic of upper respiratory tract infection and 8 symptomatic of lower respiratory tract infection), the negative rate of nasopharyngeal swab virus in patients on day 6: 100% were treated with hydroxychloroquine and Azithromycin; 57.1% of the patients only received hydroxychloroquine monotherapy; The control group was 12.5% (P < 0.001), and the drug efficacy of symptomatic patients was more significant than that of asymptomatic patients.
The biggest improvement of this clinical trial and the previous one is that it is found that the efficiency of virus elimination is significantly higher when combined with macrocyclic lipid antibiotic azithromycin. It even turns negative on the third day after enrollment, and completely turns negative on the sixth day, which is much shorter than the time reported in the previous Literature (covid-19, the average time of virus turning negative in Chinese patients is 20 days, The longest duration is even 37 days).
The following item [7-12] is a relatively new list of drugs developed specifically for the treatment of COVID-19 infection. [7-11] most of them are biological agents and injection preparations. They usually require cold chain transportation and storage. 12, oral preparations can be transported and stored at room temperature.
7、Ronapreve(Casirivimab&Imdevimab)Treatment&PEP
Casirivimab & imdevimab is called regen-cov in the United States and ronapreve in other countries and regions.
On 2021 07, 20, the MHLW approved Ronapreve to treat patients with mild to moderate COVID-19 infection via intravenous infusion.
On July 30, 2021, FDA of the United States updated the regen-cov emergency use authorization (EUA) for covid-19 antibody cocktail therapy: people with high risk of developing into severe covid-19 were given post exposure prophylaxis (PEP). These people were not fully vaccinated or were not expected to produce sufficient immune response to the vaccination, and were exposed to sars-cov-2 infected people Or because the infection occurs in the same institutional environment (such as nursing home or prison), the risk of contact with infected persons is high.
Regen-cov was administered once a month to those who needed repeated administration due to continuous exposure.
8、Remdesivir:After the outbreak, first new drugs were specially designed for the treatment of COVID-19.
Veklury (remdesivir) is invented by the antiviral Institute of Gilead based company in the United States. Veklury has broad spectrum antiviral activity in animal and in vitro, and can fight against many new viral pathogens, including Ebola virus, SARS virus, Marburg virus, Middle East respiratory syndrome and SARS-CoV-2 virus causing New Coronavirus pneumonia.
On October 8, 2020 (US time), the New England Journal of Medicine (NEJM) published the final results of the phase 3 actt-1 clinical study of the National Institute of allergy and infectious diseases (NIAID). This is a double-blind, placebo-controlled trial to study Gillie's Science in the development of antiviral drug Reed Veklury for adults hospitalized with mild or severe New Coronavirus pneumonia. On the basis of the preliminary results published on NEJM in May 2020, the final results of the ACTT-1 trial showed that in the multiple outcome assessments for New Coronavirus pneumonia, Reed's showed a consistent and clinically significant benefit compared with placebo. The final results of the trial showed that the recovery time of patients treated with redcivir was faster than previously reported.
There are nearly 30 pharmaceutical factories around the world producing general-purpose remdesivir.
9、Sotrovimab
Formerly known as vir-7831 or gsk4182136, it is a single dose monoclonal antibody against sars-cov-2. It targets the conserved epitope of "spike" protein, which is unlikely to mutate over time (which may make drug resistance more difficult). Sotrovimab combines xencor's xtend technology and is also designed to achieve high concentration in the lungs to ensure optimal penetration into the airway tissue affected by sars-cov-2 and has an extended half-life. Preclinical data show that it may not only block the virus from entering healthy cells, but also remove infected cells.
On May 26, 2021, GlaxoSmithKline and vir biotechnology announced that the U.S. FDA granted sotrovimab emergency use authorization (EUA) for the treatment of mild to moderate covid-19 adult and pediatric patients (aged 12 and over, weighing at least 40 kg). These patients tested positive for sars-cov-2 virus, And has a high risk of progression to severe covid-19, including hospitalization or death.
10、Etesevimab (LY-CoV555)
Etesevimab is a recombinant human monoclonal neutralizing antibody. It binds to sars-cov-2 surface spike protein receptor binding domain with high affinity and specificity, and can effectively block the binding between the virus and host cell surface receptor ACE2. The R & D team introduced point mutation into natural human IgG1 antibody to remove adverse effects such as tissue damage.
11、Bamlanivimab (JS016 or LY-CoV016)
Bamlanivimab is a powerful and neutralizing IgG1 monoclonal antibody against sars-cov-2 spike protein. Its research and development aims to prevent the virus from attaching and entering human cells, so as to neutralize the virus and potentially prevent and treat covid-19. Bamlanivimab is an antibody identified from a blood sample collected from the first covid-19 rehabilitation patients in the United States.
On February 9, 2021 (US Eastern time), Eli Lilly and company announced that the US FDA had granted neutralizing antibody combination therapy: bamlanivimab (ly-cov555) 700 mg and etesevimab (js016 or ly-cov016) 1400 mg emergency use authorization (EUA) for the treatment of mild and moderate covid-19 patients aged 12 and over with high risk of progression to severe or hospitalization.
Bamlanivimab alone has been authorized in several countries, while the joint programme of bamlanivimab and etesevimab has been authorized for emergency use in the United States and Italy.
12、Favipiravir(Oral tablet)
Favipiravir research and development code T-705, developed by FUJIFILM Toyama Chemical Co., Ltd., FFTC, Japan, is an antiviral drug for the treatment of drug-resistant influenza in Japan. It is being studied for the treatment of other types of viral infections, including Ebola virus (Ebola virus), COVID-19 (SARS-COV-2), Lhasa virus (T-705), and Toyama. Middle East respiratory syndrome (mers COV).
In 2020 06, Reed and Remdesivir (favipiravir) were approved in India for the treatment of severe acute coronavirus (COVID-19) infection.
The usual course of treatment for adults with favipiravir is 5 days. 1600mg once on the first day, twice a day; From day 2 to day 5, 600 mg once, twice a day.
There are nearly 100 pharmaceutical factories in the world to produce the general version of favipiravir.
COVID-19 infection causes the body's immune system to attack itself, causing inflammation and inflammation. Patients need to use immunosuppressive agents to fight inflammation.
1.Convalescent plasma
It is suitable for patients with rapid disease progression, severe and dangerous severe.
2.Intravenously injected covid-19 human immunoglobulin
It can be used in emergency for ordinary and severe patients with rapid disease progression. The recommended dosage is 20ml for ordinary type and 40ml for heavy type, which can be infused again every other day according to the improvement of the patient's condition, and the total number of times shall not exceed 5.
3、Actemra (Tocilizumab)
Tocilizumab can be used in patients with extensive lesions of both lungs and severe patients, and the level of IL-6 is increased in the laboratory. Specific usage: the first dose is 4 ~ 8mg / kg, the recommended dose is 400mg, 0.9% normal saline is diluted to 100ml, and the infusion time is more than 1 hour; For those with poor curative effect for the first time, they can be applied 14 times after the first dose is applied 12 hours (the dose is the same as the previous), the cumulative number of administration is up to 2 times, and the maximum dose of a single dose is not more than 800mg. Pay attention to allergic reaction. It is forbidden for those with active infection such as tuberculosis.
On 06 2021, 25, Roche announced that the United States FDA granted its intravenous IL-6 receptor inhibitor Actemra/RoActemra (tocilizumab) emergency use authorization (EUA) to treat adults hospitalized with COVID-19 (COVID-19) infection and children aged two and over. These patients are receiving systemic corticosteroids and require adjuvant oxygen supply, noninvasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
4、ALZUMAb(Itolizumab)
Itolizumab is an immunomodulatory anti-cd6 IgG1 monoclonal antibody. It was approved to market in India in 2013. It is also the first anti-cd6 monoclonal antibody in the world.
On 2020, 07, 10, Bicon, India biopharmaceutical company, announced that the India Drug Administration (DCGI) had approved ALZUMAb (Itolizumab) 25mg/5mL intravenous preparation for the treatment of moderate to severe acute respiratory distress syndrome (ARDS) caused by New Coronavirus pneumonia (COVID-19) and treated with cytokine release syndrome (CRS).
5、Baricitinib (Oral tablet)
Baricitinib is a JAK inhibitor, which is widely used in the treatment of various inflammatory diseases.
On July 29, 2021, the US FDA revised the emergency use authorization (EUA) of baricitinib, and now authorizes baricitinib to be used alone in the treatment of hospitalized adults and pediatric covid-19 patients aged 2 or above who need auxiliary oxygen supply, noninvasive or invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO).
6、Tofacitinib(Oral tablet)
Tofacitinib, a JAK inhibitor, has not been approved or authorized for the treatment of patients with covid-19.
On July 30, 2021, the results of the phase 3 clinical trial stop-covid (nct04469114) of oral JAK inhibitor tofacitinib in the treatment of covid-19 pneumonia inpatients were published in the International Medical Journal New England Journal of Medicine (NEJM).
The results showed that tofacitinib significantly reduced the risk of death or respiratory failure in hospitalized patients with covid-19 compared with placebo.
Treatment principle: actively prevent and treat complications, treat basic diseases, prevent secondary infection, and provide organ function support in time.
1.Glucocorticoid therapy: Methylprednisolone
For patients with progressive deterioration of oxygenation index, rapid imaging progress and excessive activation of body inflammatory response, glucocorticoids should be used in a short term (generally recommended for 3 ~ 5 days, no more than 10 days). The recommended dose is equivalent to 0.5 ~ 1mg / kg / day of methylprednisolone. It should be noted that a large dose of glucocorticoids due to immunosuppressive effect, It may delay the clearance of the virus.
2.Respiratory support: oxygen production and respiratory equipment are required
Most ordinary families do not have the above equipment and rescue conditions, which are for reference only.
If you need to use the contents of this section and believe that the patient is already in the "desert of medical resources", please believe that you can survive.
When the patient has a high fever (temperature above 38.5 °C ), symptomatic treatment.
1 .Gypsum
If there is gypsum, boil 15 ~ 30g gypsum with water, filter out the gypsum, take the liquid, once in the morning and once in the evening (40 minutes after meals), take it gently, and take it for 3 days. If the symptoms improve but do not recover, continue to take it for the second course of treatment.
If the patient has slight fever, the dosage of gypsum should be small, and the fever symptoms are strong. The dosage of gypsum can be increased.
2 .Radix Bupleuri
Ingredients in some plants can promote perspiration and lower body temperature, such as taking Radix Bupleuri extract juice.
3. Physical cooling
If there is no cooling medicine, wet the hot water with a towel (the water temperature is about 40°C) and wipe the armpits, forehead, soles of feet, palms and other parts, which can play a physical cooling effect.
The components in some plants have the function of inhibiting bacteria and viruses. They can be extracted and taken with liquid to fight bacteria and viruses, such as:
Components in some plants have immunosuppressive function and can be extracted and taken with liquid to fight inflammation, such as:
Mash the plant, soak in water for 30 minutes, filter out impurities and take it.
If the patient has sputum:
The best option is to receive treatment in the hospital. The second is to buy household oxygen generator. Finally, electrolytic water is relatively dangerous for people without technical knowledge. Usually, it may be difficult for families to make enough oxygen, and the international minimum medical oxygen concentration standard is more than 82%.
If the patient is critically ill, be sure to make oxygen by yourself, please follow the following technical points:
For a more detailed operation guide, you can search for self-made oxygen tutorials and videos on the Internet.
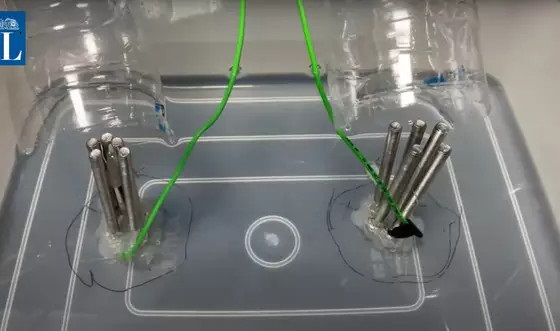
Electrolytic water oxygen production
The document version number consists of five parts:
Different language versions are as follows:
Content iteration: the content of the document will be continuously updated with the progress of the medical community.